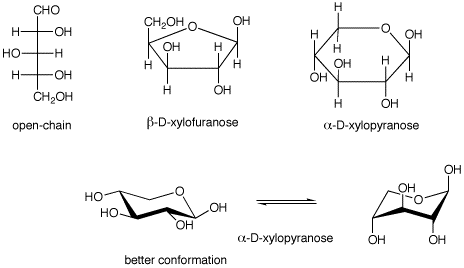
Other principal conformations of pyranoses are halfchair H boat B and skew S conformation which are named as indicated. The resulting UDP-4-keto-pentose adopts a flattened 4 C 1 chair Fig. xylose chair conformation.
Xylose Chair Conformation, The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. Because many compounds feature structurally similar six-membered rings. A Structure of the WT XylFII-LytSN complex with bound D-xylose XylFII-LytSN-xyl.

Draw the second chair conformation ring-flip-check this post if not sure. Other principal conformations of pyranoses are halfchair H boat B and skew S conformation which are named as indicated. Number the ring and draw any chair conformation of the compound.
On the basis of A1 Get Best Price Guarantee.
The detection of xylose cyclic phosphonate as the turnover product reveals significant new details about the AXS-catalyzed reaction and supports the proposed retroaldol-aldol mechanism of catalysis. The UDP-xylose product is in a relaxed 4 C 1 conformation. Draw the second chair conformation ring-flip-check this post if not sure. 252 It is noteworthy that the complex with the corresponding lactam 121 Scheme 30. Lowest energy conformations have the largest nonhydrogen subsstituents occupying the lowest energy equatorial position. Number the ring and draw any chair conformation of the compound.
Another Article :
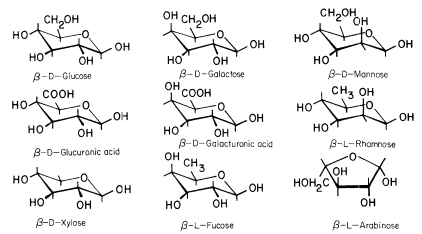
These structures make it easy to show the configuration at each stereogenic center in the molecule without using wedges and dashes. For most of the compounds studied the spectra are consistent with the predominance of the C1 conformation of the pyranose ring but there is evidence of some departures from the ideal chair shape. For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. And now the stabilities. B acetic anhydride in pyridine b D-galactose b D-allose. Quiz 25.

The resulting UDP-4-keto-pentose adopts a flattened 4 C 1 chair Fig. In organic chemistry cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. A trajectory initiated for the wild-type enzymesubstrate complex with the proximal xylose ring bound at the 1 subsite adjacent to the scissile glycosidic bond in the 4 C 1 chair conformation shows spontaneous transformation to the 25 B boat conformation and potential of mean force calculations indicate that the boat is 30 kJ mol 1 lower in free energy than the chair. And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. Lets get some practice drawing it chair confirmations one way to do it is to start by drawing two parallel lines that are offset from each others let me go ahead and show you what I mean so heres heres one line and then here is another line theyre parallel to each other but theyre offset a little bit next were going to draw two horizontal dotted lines so the top horizontal dotted line is going to be on level with that top. Synthesis Of 2 3 O Benzyl Ribose And Xylose And Their Equilibration Sciencedirect.

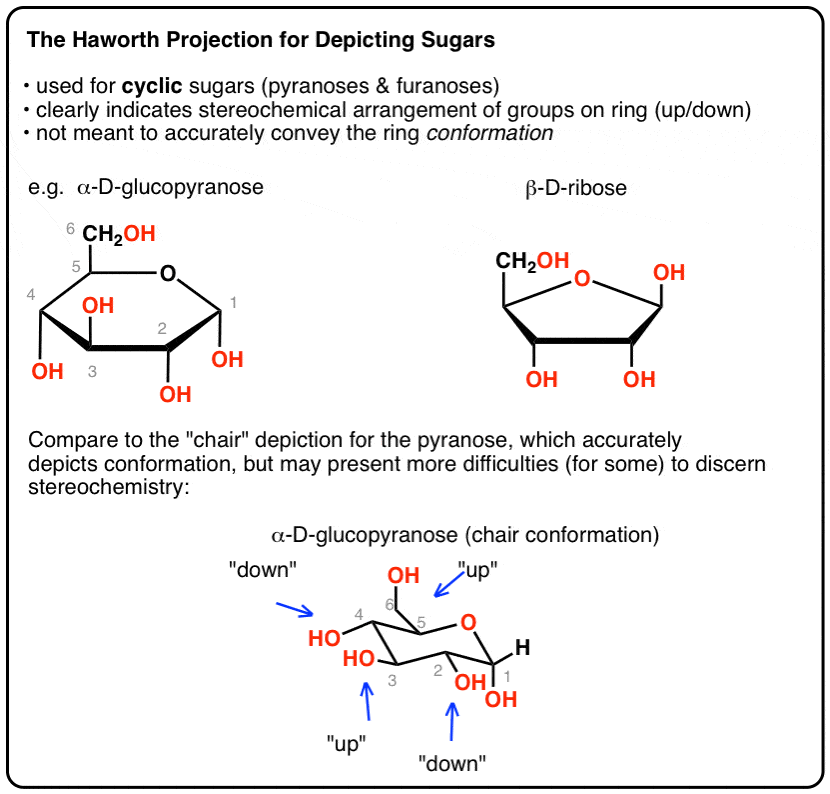
Hydrogen atoms in axial positions are shown in red while those in equatorial positions are in blue. Abstract and Figures. The detection of xylose cyclic phosphonate as the turnover product reveals significant new details about the AXS-catalyzed reaction and supports the proposed retroaldol-aldol mechanism of catalysis. Lowest energy conformations have the largest nonhydrogen subsstituents occupying the lowest energy equatorial position. Draw the chair conformation of the following. Tiedosto D Xylose Svg Wikipedia.

252 It is noteworthy that the complex with the corresponding lactam 121 Scheme 30. For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. Number the ring and draw any chair conformation of the compound. The UDP-xylose product is in a relaxed 4 C 1 conformation. Fischer Projections Haworth Structures and Chair Conformers The acyclic structure of a sugar is commonly drawn as a Fischer projection. Open And Cyclic Form Of D Xylose With 4 C1 And 1 C4 Chair Download Scientific Diagram.
For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. Draw the 2 chair conformation of the following pyranose forms of beta d mannose alpha D xylose beta D galactose alpha D fructose and identify which of the conformations has the lowest energy. Arg277 which is positioned by a salt-link interaction with Glu120 closes up the catalytic site and prevents release of the UDP-4-keto-pentose and NADH intermediates. B acetic anhydride in pyridine b D-galactose b D-allose. Left Side view of the. The Molecular Biology Of Plant Cells D0e311.

Do you mean D-xylose. Given the following Haworth projection of eqbeta-D-Glucopyranose eq. The data suggest that this observation is unlikely to be due to an unfavorable equilibrium but rather results from substrate inhibition by the most stable chair conformation of UDP-D-xylose. PDB 1OD8 was discovered to feature the acidbase catalytic residue Glu128 in the deprotonated state whereas the nucleophile Glu 236 was found in protonated. A B-D - galactopyranose b B-D mannopyranose 7. L Arabinose Binding Isomerization And Epimerization By D Xylose Isomerase X Ray Neutron Crystallographic And Molecular Simulation Study Structure.

Left Side view of the. The chair is by far the most stable and only the skew conformation has an energy minimum in a similar range but this is still some 20 kJ higher than the chair. The UDP-xylose product is in a relaxed 4 C 1 conformation. In organic chemistry cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. The detection of xylose cyclic phosphonate as the turnover product reveals significant new details about the AXS-catalyzed reaction and supports the proposed retroaldol-aldol mechanism of catalysis. A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram.

The UDP-xylose product is in a relaxed 4 C 1 conformation. It is concluded that for aldopyranoses in a chair conformation the chemical shift of equatorial protons at a given position is virtually independent of configurational changes at other positions. The detection of xylose cyclic phosphonate as the turnover product reveals significant new details about the AXS-catalyzed reaction and supports the proposed retroaldol-aldol mechanism of catalysis. Given the following Haworth projection of eqbeta-D-Glucopyranose eq. A trajectory initiated for the wild-type enzymesubstrate complex with the proximal xylose ring bound at the 1 subsite adjacent to the scissile glycosidic bond in the 4 C 1 chair conformation shows spontaneous transformation to the 25 B boat conformation and potential of mean force calculations indicate that the boat is 30 kJ mol 1 lower in free energy than the chair. Scheme 1 Fisher Projection Of D Xylose Centre And Haworth Projections Download Scientific Diagram.
And by Mohr 13 namely the chair and boat forms could be extended to pyranoid sugars. Besides its applications in bioenergy and biosynthesis β-D-xylose is a very simple monosaccharide that exhibits relatively high rigidity. For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. 2- what are the chair conformation for D-xylulose. Arg277 which is positioned by a salt-link interaction with Glu120 closes up the catalytic site and prevents release of the UDP-4-keto-pentose and NADH intermediates. The Haworth Projection Master Organic Chemistry.

A cyclohexane molecule in chair conformation. For each chair conformer add the energy of all the groups on axial position. The C2-C3-C5-O square forms a plane whereas the C1 and C4 atoms take up a higher or lower position thus yielding the two possible chairs 1C 4 and 4C 1. 252 It is noteworthy that the complex with the corresponding lactam 121 Scheme 30. By the NTD and CTD of XylFII the pyranose ring of the D-xylose molecule adopts a chair conformation and residues from both ter-minal domains of XylFII form hydrophobic and hydrogen-bonding Fig. Scheme 1 Fisher Projection Of D Xylose Centre And Haworth Projections Download Scientific Diagram.
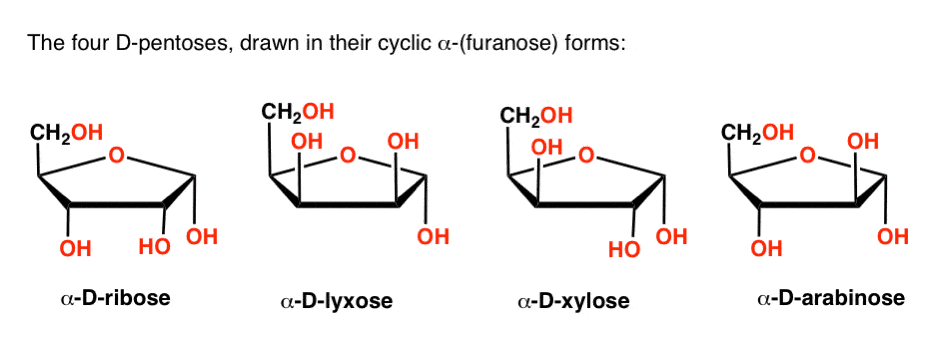
The UDP-xylose product is in a relaxed 4 C 1 conformation. For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. Number the ring and draw any chair conformation of the compound. For each chair conformer add the energy of all the groups on axial position. A trajectory initiated for the wild-type enzymesubstrate complex with the proximal xylose ring bound at the 1 subsite adjacent to the scissile glycosidic bond in the 4 C 1 chair conformation shows spontaneous transformation to the 25 B boat conformation and potential of mean force calculations indicate that the boat is 30 kJ mol 1 lower in free energy than the chair. The Haworth Projection Master Organic Chemistry.
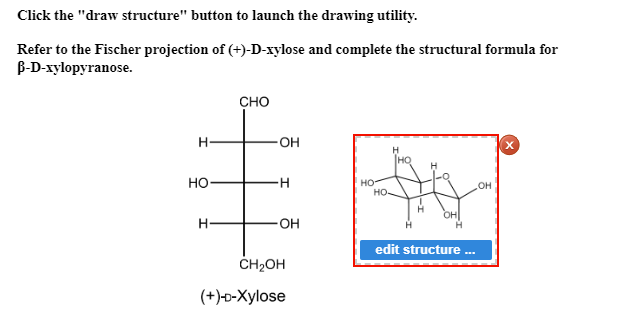
And now the stabilities. As such it provides the best. PDB 1OD8 was discovered to feature the acidbase catalytic residue Glu128 in the deprotonated state whereas the nucleophile Glu 236 was found in protonated. Hydrogen atoms in axial positions are shown in red while those in equatorial positions are in blue. B acetic anhydride in pyridine b D-galactose b D-allose. Solved Click The Draw Structure Button To Launch The Chegg Com.
Do you mean D-xylose. 1- What is the alpha and beta haworth conformation of D-xylulose. Determination of the anomeric configuration of D-xylose with D-xylose isomerases. 252 It is noteworthy that the complex with the corresponding lactam 121 Scheme 30. What is the full name of the monosaccharide shown below. 2.

For each chair conformer add the energy of all the groups on axial position. A trajectory initiated for the wild-type enzymesubstrate complex with the proximal xylose ring bound at the 1 subsite adjacent to the scissile glycosidic bond in the 4 C 1 chair conformation shows spontaneous transformation to the 25 B boat conformation and potential of mean force calculations indicate that the boat is 30 kJ mol 1 lower in free energy than the chair. Kersters-Hilderson H Claeyssens M van Doorslaer E de Bruyne CK. What is the full name of the monosaccharide shown below. For α-glucose β-glucose and β-mannose 729 rotamers were generated for each pucker resulting in 27 702 conformations for each of these six-carbon monosaccharides. A Equatorial And Axial Directions Of The Ring Shown With E And A Download Scientific Diagram.
On the basis of A1 Get Best Price Guarantee. Like the cyclohexane ring adopt a low-energy conformation that looks like a chair. Draw the 2 chair conformation of the following pyranose forms of beta d mannose alpha D xylose beta D galactose alpha D fructose and identify which of the conformations has the lowest energy. Draw the second chair conformation ring-flip-check this post if not sure. It is concluded that for aldopyranoses in a chair conformation the chemical shift of equatorial protons at a given position is virtually independent of configurational changes at other positions. 2.









