Carefully study this molecule by rotating it on your screen. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. lowest chair conformation.
Lowest Chair Conformation, The higher energy chair conformation contains two axial methyl groups. You can see the chair conformation of cyclohexane in the image to the right. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy.
 Chapter 3 Alkanes And Cycloalkanes Conformations And Cistrans From slidetodoc.com
Chapter 3 Alkanes And Cycloalkanes Conformations And Cistrans From slidetodoc.com
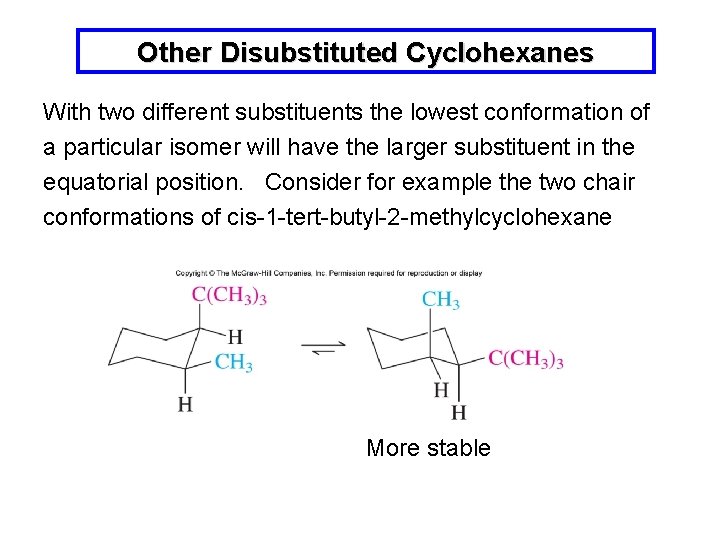
E The lower energy chair conformation contains two axial methyl groups. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a chair. Steric parameters are tabulated in many organic chemistry textbooks the most common of which is the A-value.
Chair Conformations Examples - YouTube.
You can see the chair conformation of cyclohexane in the image to the right. D The higher energy chair conformation contains two axial methyl groups. 1 if both methyl groups. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. If they occupy the axial positions then it will be the highest energy chair conformation because of. For each chair conformer add the energy of all the groups on axial position.
Another Article :
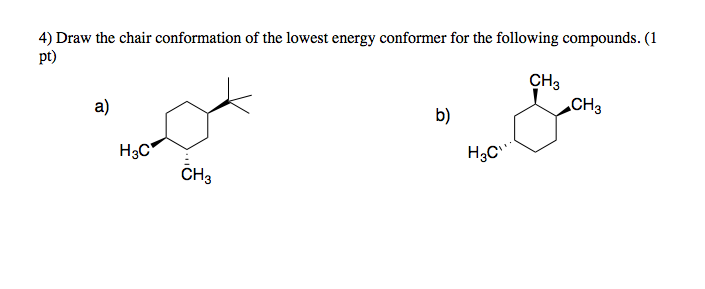
The chair conformation is the most stable conformation of cyclohexane. Like in given figure no. In chemistry conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. The different conformations are called conformers a blend of the words conformation and isomer. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation Part A Draw l-ethyl-4-methylcyclohexane in its lowest energy conformation 1. Solved Chair Conformation Of The Lowest Energy Conformer Chegg Com.
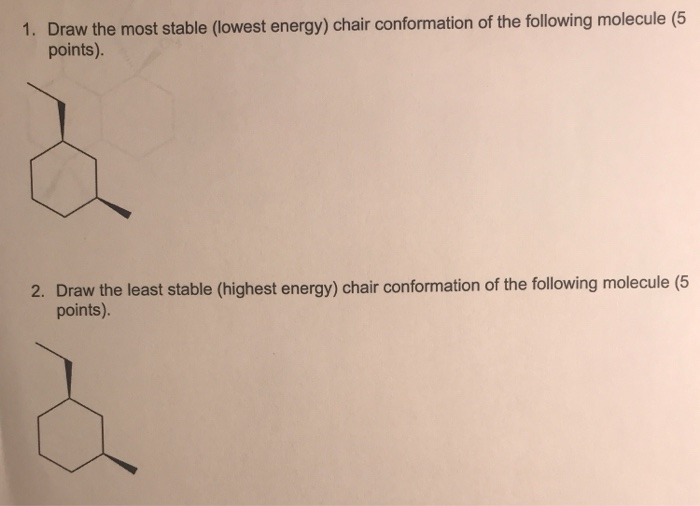
Carefully study this molecule by rotating it on your screen. The chair conformation is the most stable conformer. E The lower energy chair conformation contains two axial methyl groups. In chemistry conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. Solved 1 Draw The Most Stable Lowest Energy Chair Chegg Com.

In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Choose a chair from the Templates toolbar at the bottom. This means that the chair conformation is the structure that is observable. The different conformations are called conformers a blend of the words conformation and isomer. The chair conformation is the most stable conformation of cyclohexane. The Four Lowest Energy Structures Of Morpholine Calculated By Download Scientific Diagram.
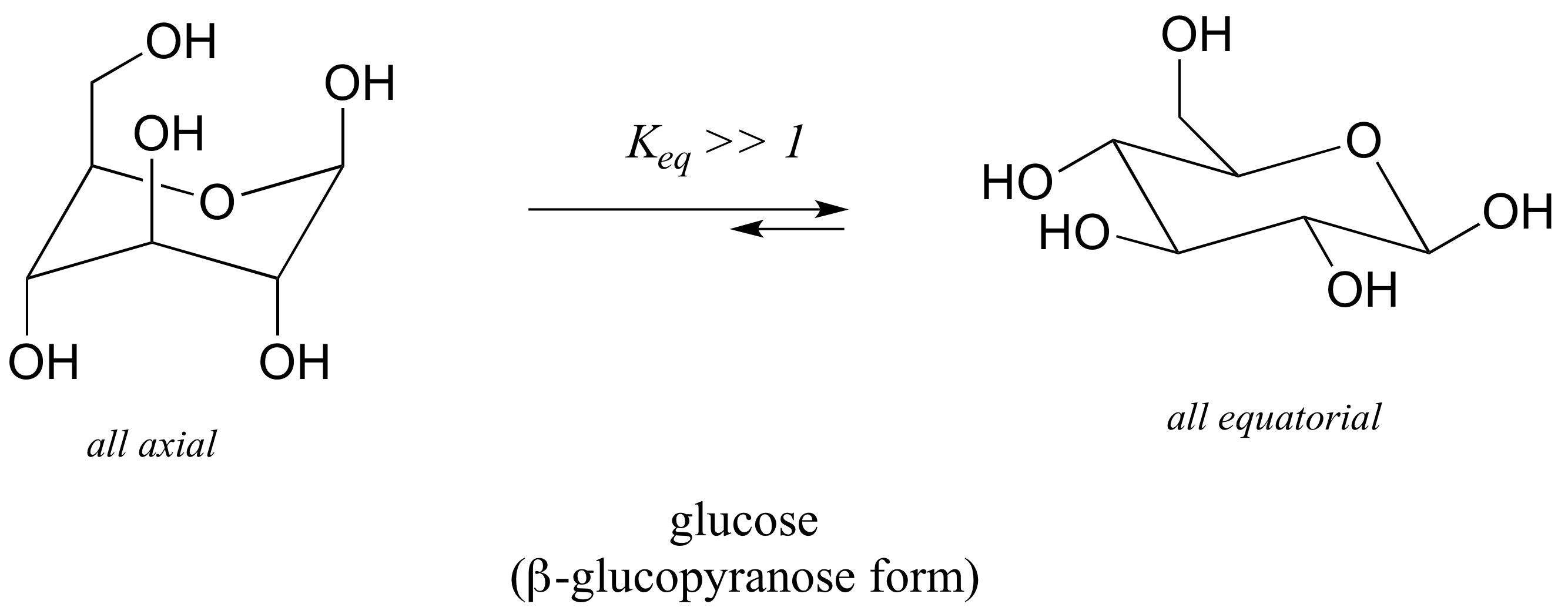
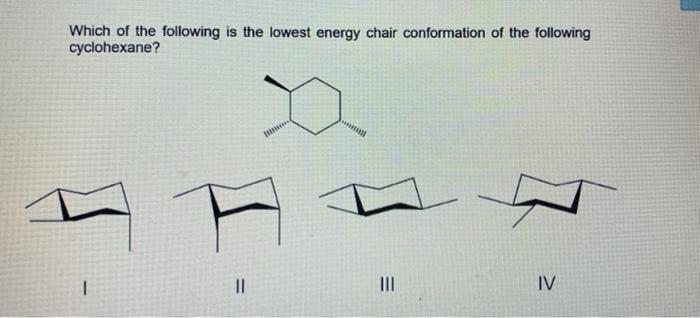
To determine the chair conformation of a hexose it is generally easiest to draw it and compare it with β-D-glucose where all heavy groups are equatorial and the conformation is 4 C 1. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. I am asked to draw the most stable chair conformation of compound 1. Which of the following is the lowest energy chair conformation of the following cyclohexane. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. 3 2 Conformations Of Cyclic Organic Molecules Chemistry Libretexts.
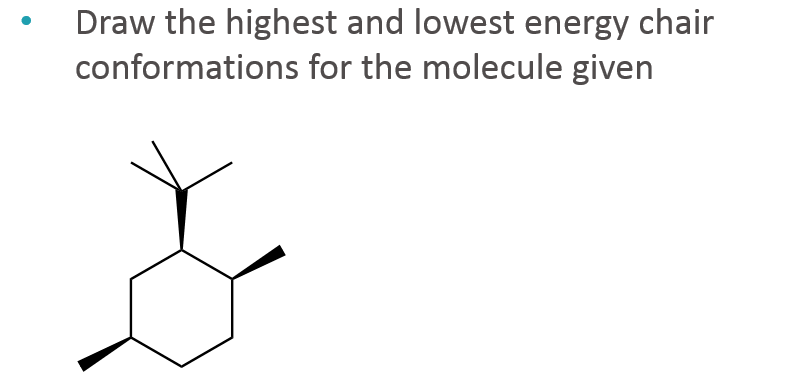
The lower energy chair conformation contains two axial methyl groups. Steric parameters are tabulated in many organic chemistry textbooks the most common of which is the A-value. Notice that half of the hydrogen atoms have a different chemical environment. E The lower energy chair conformation contains two axial methyl groups. The other conformations are known as the twist and boat forms. Solved Draw The Highest And Lowest Energy Chair Chegg Com.
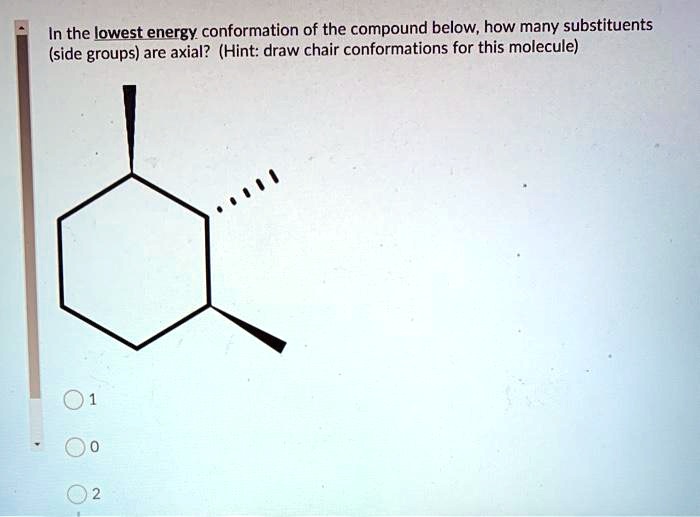
14 Which of the statements below correctly describes the chair conformations of trans-13-. D The higher energy chair conformation contains two axial methyl groups. A The two chair conformations are equal in energy. Chair Conformations Examples - YouTube. Notice that half of the hydrogen atoms have a different chemical environment. Zxiiojjrv87j3m.
This is your first axial substituent. 10 Jul2017 Tutor. 14 Which of the statements below correctly describes the chair conformations of trans-13-. Choose a chair from the Templates toolbar at the bottom. 1 if both methyl groups. Solved Which Of The Conformers Is The Lowest Energy Chair Chegg Com.

10 Jul2017 Tutor. Chair Conformations Examples - YouTube. Draw the second chair conformation ring-flip-check this post if not sure. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. Solution Draw The Lowest Energy Chair Co Organic Chem.

The chair conformation is the most stable conformation of cyclohexane. As we have discussed earlier the chair conformation of cyclohexane is the most stable because it has the lowest strain energy. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. What is the lowest energy chair conformation. The lower energy chair conformation contains two axial methyl groups. Three Of The Lowest Minimum Energy Conformations Of Cyclooctane Download Scientific Diagram.

An alternate conformation for a six-membered ring is called the boat. The higher energy chair conformation contains two axial methyl groups. A The two chair conformations are equal in energy. Identify the up tip OR down tip of your chair conformation and draw a straight line up up tip or down down tip parallel to the y-plane. Draw the second chair conformation ring-flip-check this post if not sure. Evaluating Relative Stability Of Chair Conformers Youtube.
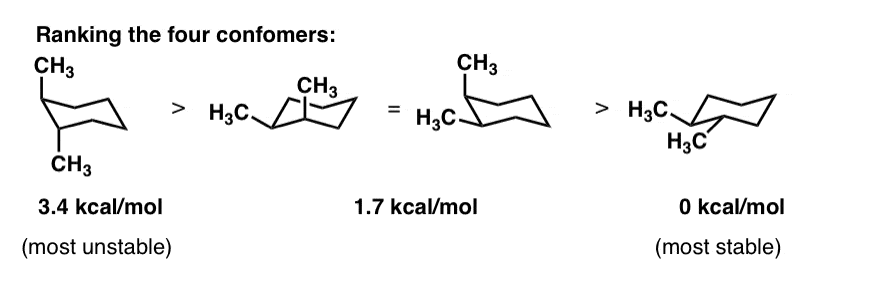
A The two chair conformations are equal in energy. Lowest energy form is the most important. Draw the second chair conformation ring-flip-check this post if not sure. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. In the given figure various possible chair conformations of 12-dimethylcyclohexane are drawn. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. The lowest energy conformer for a cyclohexane ring with multiple substituents will be the one in which the largest group occupies an equatorial position. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Solved Which Of The Following Is The Lowest Energy Chair Chegg Com.

For cis-13-dimethylcyclohexane the lowest energy chair conformation implies that the methyl groups of 1 and 3 carbons of the cyclohexane ring must occupy the equatorial positions. What is the lowest energy chair conformation. The depiction below on the left has the red-colored hydrogen atoms in what is termed axial conformation. Draw cis-1-ethyl-4-methylcyclohexane in its lowest energy conformation Part A Draw l-ethyl-4-methylcyclohexane in its lowest energy conformation 1. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. Rules For Naming Alkanes Organicchemistry Orgo Ochem Mcat Chemistry Lessons Organic Chemistry Organic Chemistry Study.

Carefully study this molecule by rotating it on your screen. Cyclohexane is unique in being the only cyclic hydrocarbon which is. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations conformations that correspond to local minima on the potential energy surface are specifically called. This chair conformation is the lowest energy conformation for cyclohexane and other six-membered rings. The chair conformation has alternating axial up axial down so once you have that single axial substituent move on to. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
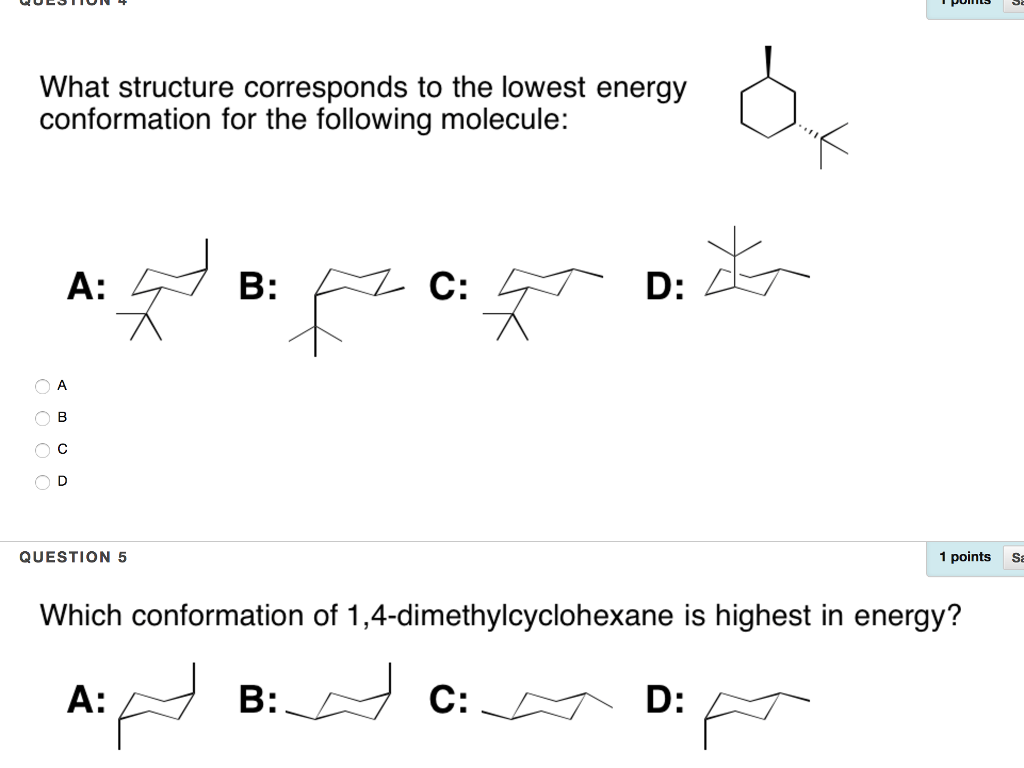
The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. In this conformation there is no torsional strain at all and as we shall see later no strain of any kind. 10 Jul2017 Tutor. You can see the chair conformation of cyclohexane in the image to the right. The lower energy chair conformation contains one axial methyl group and one equatorial methyl group. Solved What Structure Corresponds To The Lowest Energy A Chegg Com.