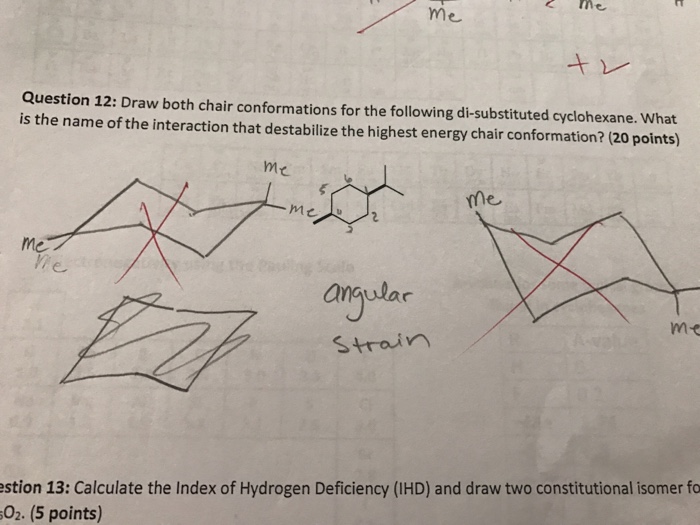
There is a severe crowding among the atoms in axial positions on the same side of the ring. When a model of a cyclohexane in a chair conformation is placed on a table note that it is standing on three of the axial hydrogens flip the model over to have it stand on the other three axial hydrogens. chair conformation hydrogen.
Chair Conformation Hydrogen, Therefore to reduce torsional strain cyclohexane adopts a three-dimensional structure known as the chair conformation. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. These H atoms are respectively referred to as axial.
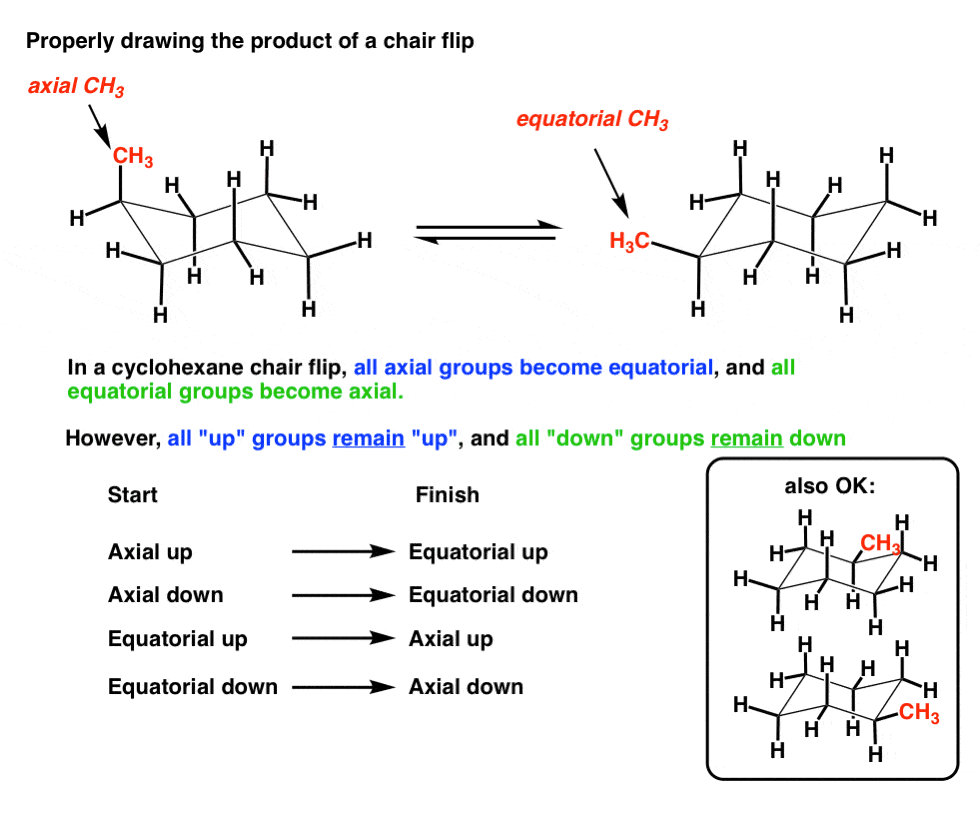
In this process the equa-torial hydrogens have become axial and the axial hydrogens have become equatorial. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. A up-axial hydrogen up-axial hydrogen down-axial hydrogen down-axial hydrogen down-equatorial hydrogen.
Therefore to reduce torsional strain cyclohexane adopts a three-dimensional structure known as the chair conformation.
This means that the chair conformation is the structure that is observable. There is a severe crowding among the atoms in axial positions on the same side of the ring. The hydrogens which radiate out from around the ring are called equatorial hydrogens. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. A up-axial hydrogen up-axial hydrogen down-axial hydrogen down-axial hydrogen down-equatorial hydrogen. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexaneThese are two different structures in which the cyclohexane molecule can exist but they have different stabilities.
Another Article :
Entirely strain-free conformation called the chair conformation in which all bond angles are perfectly tetrahedral and all bonds to hydrogen are perfectly staggered. As a result even though the rate at which these two conformations interchange is about 1 x 10 5 s -1 we can assume that most cyclohexane molecules at any moment in time are in the chair conformation. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. The equatorial bonds alternate from slightly up to slightly down in their orientation from one carbon to the next. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. The Cyclohexane Chair Flip Master Organic Chemistry.
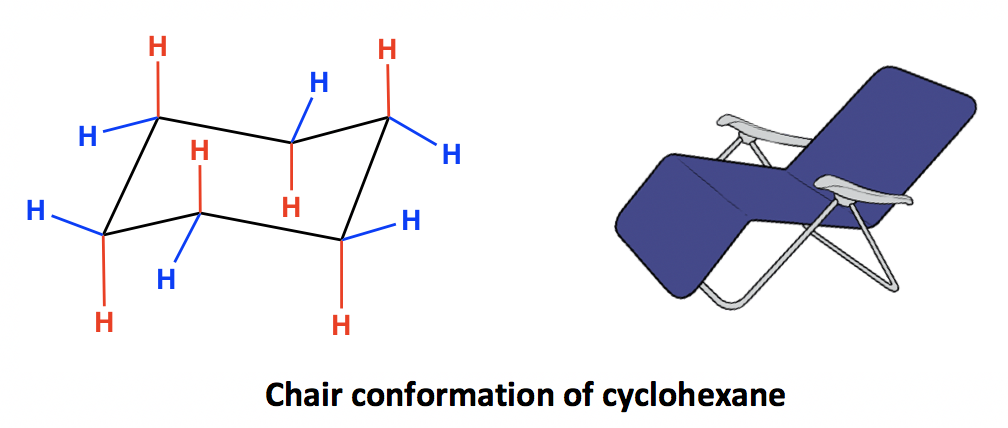
Transcribed image text. This specific conformation that we are going to look at in a moment is called the chair conformation. Hence the torsional strain in the chair conformation is small. As a result even though the rate at which these two conformations interchange is about 1 x 10 5 s -1 we can assume that most cyclohexane molecules at any moment in time are in the chair conformation. At any point on the chair that sticks up put the axial hydrogen sticking straight up. 4 3 Conformation Analysis Of Cyclohexane Organic Chemistry.

Identify the cyclohexane chair conformation with an axial ethyl substituent and equatorial methyl group. This is true for 1R-33-dichlorocyclohexanol. All carbon centers are equivalent. This specific conformation that we are going to look at in a moment is called the chair conformation. The chair conformation is free of torsional strain as well. Boat Conformation Axial Hydrogens Chemistry Stack Exchange.

This means that the chair conformation is the structure that is observable. The chair conformation is free of torsional strain as well. The chair conformation which is the most stable form solves this problem by placing hydrogen in one of two positions- equatorial or axial. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. Moreover the hydrogen atoms at opposite corners of the cyclohexane ring. E2 And E1 Elimination Reactions Of Cyclohexanes Practice Problems.
In each of the conformations drawn in step 4 circle the axial substituents other than hydrogen. Even if you are explicitly given the 2-D cyclohexane you should convert it into the 3-D chair prior to solving the E2 reaction. The terms chair conformation and boat conformation come under organic chemistry and they are mainly applicable to cyclohexaneThese are two different structures in which the cyclohexane molecule can exist but they have different stabilities. This is true for 1R-33-dichlorocyclohexanol. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Structures Stability And Hydrogen Bonding In Inositol Conformers Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C5cp02690c.

At any point on the chair that sticks up put the axial hydrogen sticking straight up. At any point on the chair that sticks down draw the axial hydrogen straight down. This specific conformation that we are going to look at in a moment is called the chair conformation. After the axial hydrogens are drawn adding in the equatorial hydrogens around the equator of the chair is a fairly straightforward task. When viewed along any carboncarbon bond viewing the structure from an end Fig2 the bonds are seen to be perfectly staggered. Boat Conformation Axial Hydrogens Chemistry Stack Exchange.
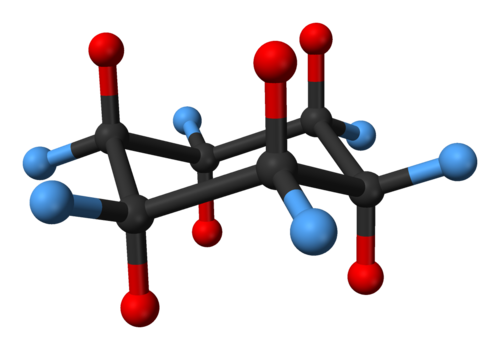
Half of the hydrogens are in the plane of the ring equatorial while the other half are perpendicular to the plane axial. At any point on the chair that sticks down draw the axial hydrogen straight down. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. Look at the axial-methylcyclohexane in chair conformation shown. Cyclohexane Conformation Wikiwand.
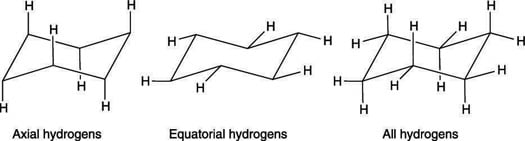
When a cyclohexane ring undergoes a chairchair conformational change a ring flip all of the bonds that were axial become equatorial and all bonds that were equatorial become axial. Identify the cyclohexane chair conformation with an axial ethyl substituent and equatorial methyl group. There is a severe crowding among the atoms in axial positions on the same side of the ring. This too is almost free of angle strain but in contrast has torsional strain associated with eclipsed bonds at the four of the C atoms that form the side of the boat. But notice what has happened to the hydrogens. How To Draw The Chair Conformation Of Cyclohexane Dummies.
At any point on the chair that sticks up put the axial hydrogen sticking straight up. 111 IV 1 O O III O IV ov Identify the correct chair conformation of cyclohexane showing all the equatorial hydrogen atoms. The difference between the energies of the chair conformation in which the hydrogen atoms are staggered and the boat conformation in which they are eclipsed is about 30 kJmol. As a result even though the rate at which these two conformations interchange is about 1 x 10 5 s -1 we can assume that most cyclohexane molecules at any moment in time are in the chair conformation. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.

At 25 C 9999 of all molecules in a cyclohexane solution adopt this conformation. A up-axial hydrogen up-axial hydrogen down-axial hydrogen down-axial hydrogen down-equatorial hydrogen. There is a severe crowding among the atoms in axial positions on the same side of the ring. This is true for 1R-33-dichlorocyclohexanol. Half of the hydrogens are in the plane of the ring equatorial while the other half are perpendicular to the plane axial. Cyclohexane Structure Formula Conformations Video Lesson Transcript Study Com.
Axial H atoms equatorial H atoms Groups in axial positions are more hindered than groups in equatorial positions. This means that the chair conformation is the structure that is observable. When a model of a cyclohexane in a chair conformation is placed on a table note that it is standing on three of the axial hydrogens flip the model over to have it stand on the other three axial hydrogens. The symmetry is D 3d. Moreover the hydrogen atoms at opposite corners of the cyclohexane ring. Rings Cis Trans And Axial Equatorial Relationships Chemistry Libretexts.
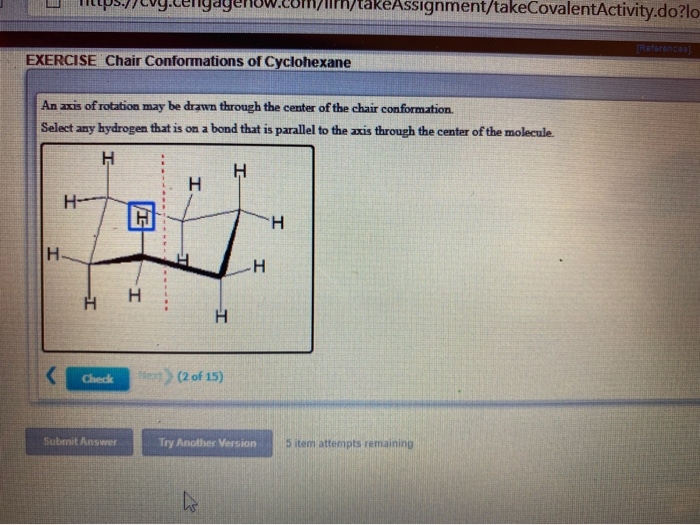
This means that the chair conformation is the structure that is observable. All carbon centers are equivalent. This specific conformation that we are going to look at in a moment is called the chair conformation. At any point on the chair that sticks up put the axial hydrogen sticking straight up. Notice how the axial-methyl group is thrown closely together with the axial hydrogens in carbons 3 and 5. Solved An Axis Of Rotation May Be Drawn Through The Center Chegg Com.
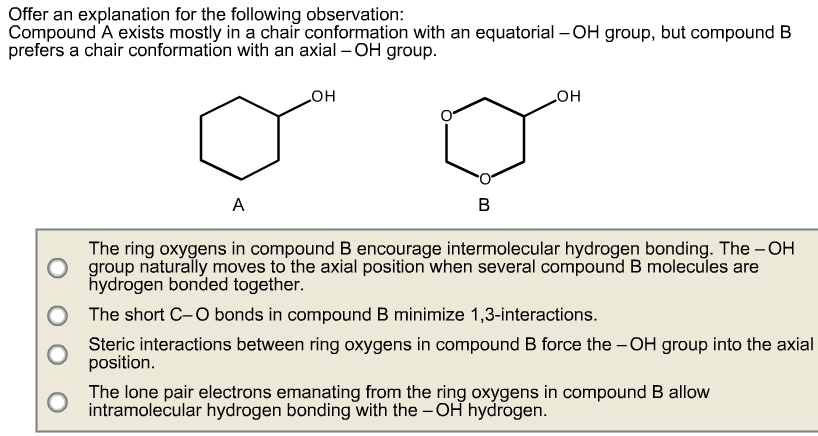
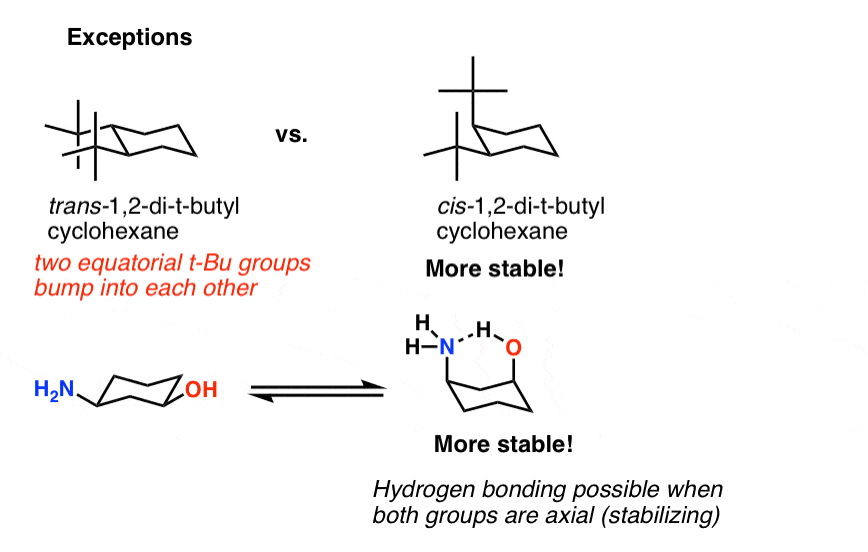
At any point on the chair that sticks down draw the axial hydrogen straight down. When dealing with E2 reactions using cyclohexane you must draw the chair conformation to see if there are any neighboring anti-periplanar hydrogens. In a chair conformation with a few exceptions only axial substituents will give rise to steric strain energy. Look at the axial-methylcyclohexane in chair conformation shown. H HH HH H II III IV 01 O II O IV OV Identify the correct chair conformations of the following compound and then indicate which one is more stable. Solved Offer An Explanation For The Following Observation Chegg Com.
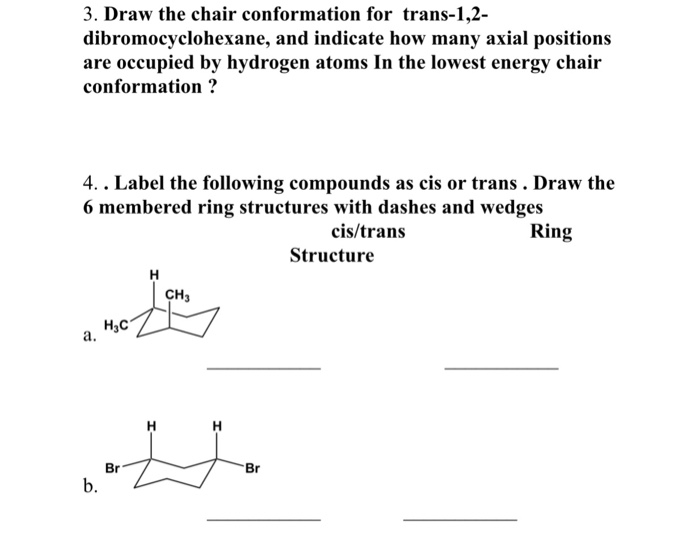
A second much less stable conformer is the boat conformation. These H atoms are respectively referred to as axial. As a result even though the rate at which these two conformations interchange is about 1 x 10 5 s -1 we can assume that most cyclohexane molecules at any moment in time are in the chair conformation. When a cyclohexane ring undergoes a chairchair conformational change a ring flip all of the bonds that were axial become equatorial and all bonds that were equatorial become axial. After the axial hydrogens are drawn adding in the equatorial hydrogens around the equator of the chair is a fairly straightforward task. Solved Draw Both Chair Conformations For The Following Chegg Com.
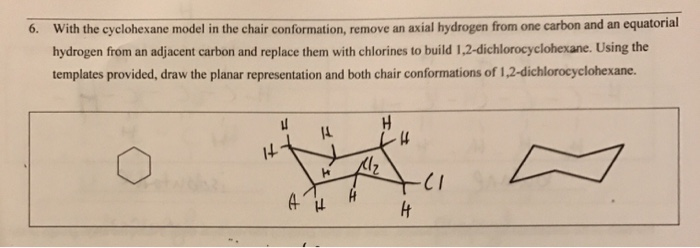
111 IV 1 O O III O IV ov Identify the correct chair conformation of cyclohexane showing all the equatorial hydrogen atoms. When dealing with E2 reactions using cyclohexane you must draw the chair conformation to see if there are any neighboring anti-periplanar hydrogens. The definition of chair conformation is the lowest energy conformation for cyclohexane in which all bond angles are fairly close to 1095 and all hydrogen atoms are staggered. The chair conformation is considered to be the lowest energy conformation because it lacks both angle strain and torsional strain. Hence the torsional strain in the chair conformation is small. Solved 6 With The Cyclohexane Model In The Chair Chegg Com.