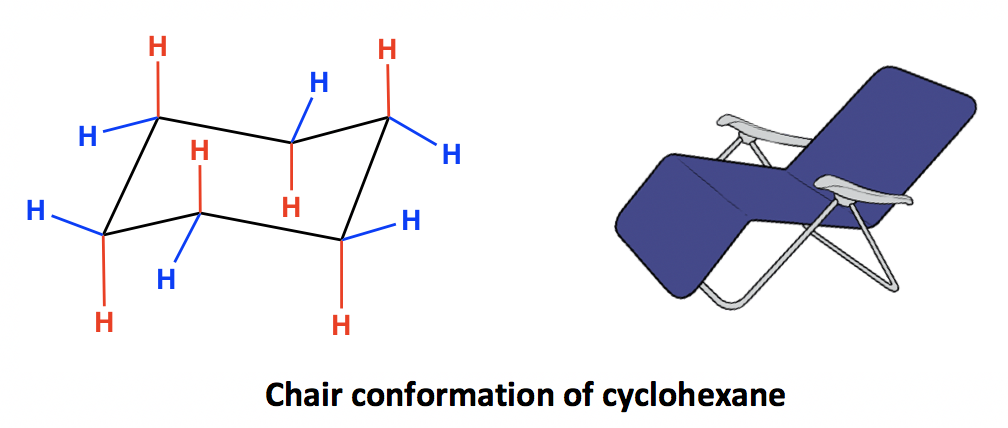
The lactam ring adopts an envelope conformation whereas the cyclohexane ring has an almost ideal chair conformation. Boat chair The boat and chair conformations are interconvertable by passing through some very unstable high energy structures called the half-chair and the twist-boat. chair conformation determination.
Chair Conformation Determination, Both compounds adopt chairenvelope conformation. From the value of dihedral angle and coupling constant you can identify whether is chair form or boat form. Cyclohexane is the chair conformation shown below.
 Cyclohexane Chair Conformation Stability Which One Is Lower Energy From masterorganicchemistry.com
Cyclohexane Chair Conformation Stability Which One Is Lower Energy From masterorganicchemistry.com
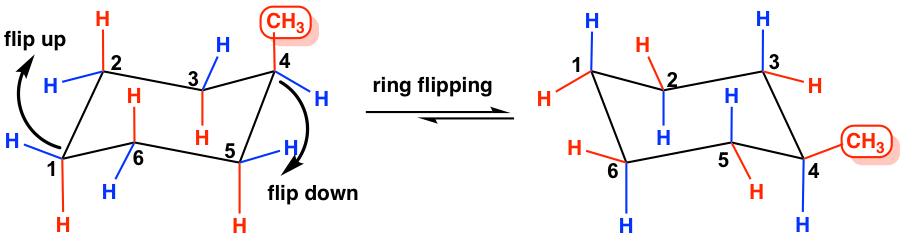
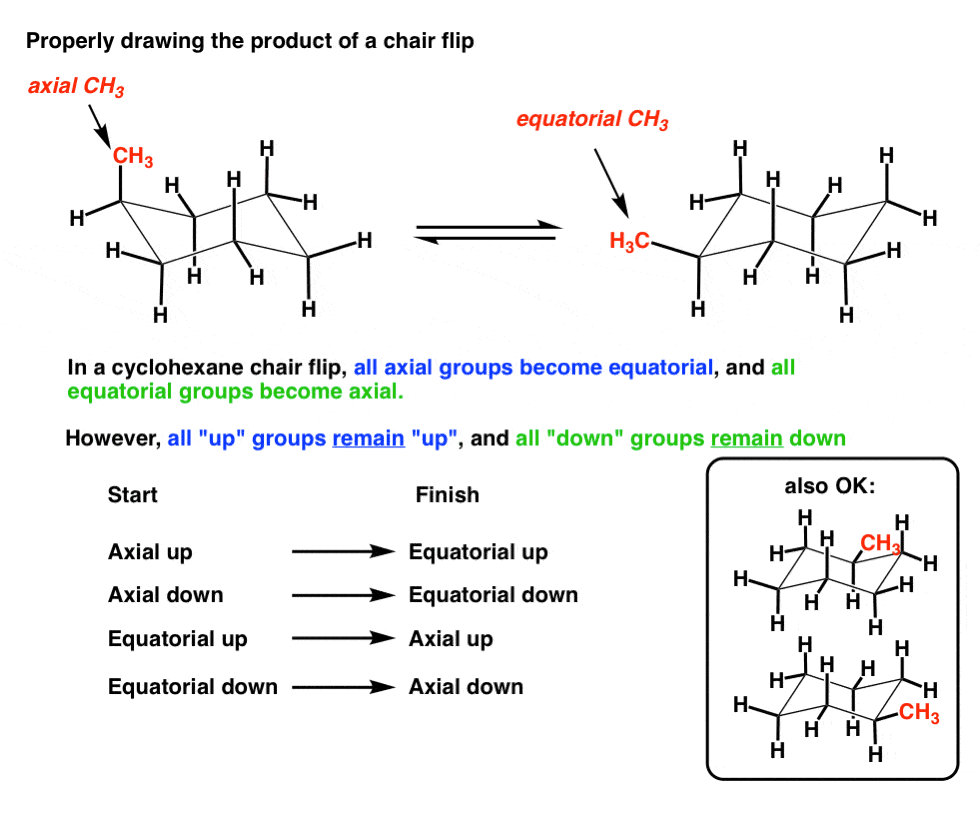
Use the steps in Figure 4-28 to practice drawing this chair conformation until you can draw it without having to refer to the figure. A flip-chair conformation is the conversion of one cyclohexane chair conformation to another by rotation around the carbon-carbon single bonds. Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation.
First thing is to number your substituents or carbon atom on the initial molecule like you did already.
The following chair conformation of cyclohexane is obtained after the one in Figure 4-28 has undergone a chair flip. Determined from the name or a 2D drawing. Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. In a chair there are three carbons that are like mountain peaks red balls and three that are notches b. Chair conformation is the term used in organic chemistry that represents the chair-like structure of a carbon ring consisting of six carbon atoms.
Another Article :

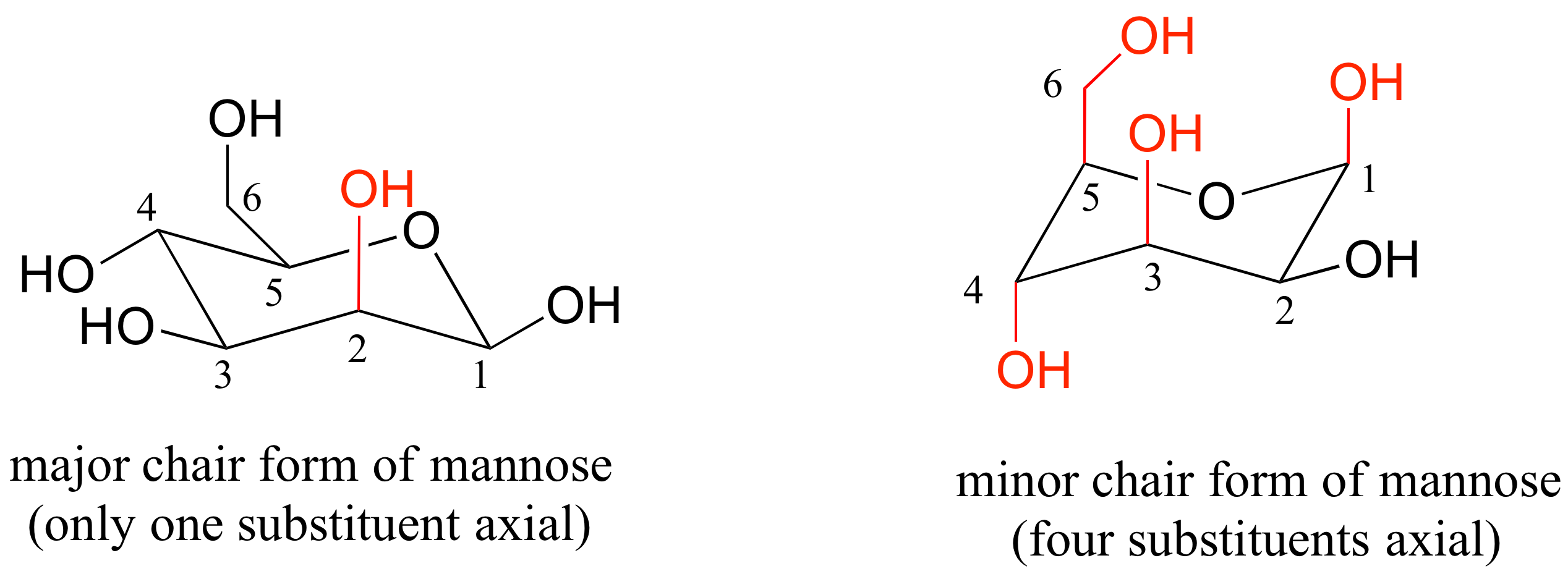
Then you should familiarize yourself with the geometry of chair conformation. Now that the molecule is in the boat conformation the energy can be determined for a boat conformation. Cyclohexane is the chair conformation shown below. Chair conformation chirality. Cyclohexane can flip between two chair conformations. Rules Of Thumb Rots For Chair Conformations And Substituent Stability Teach The Mechanism.
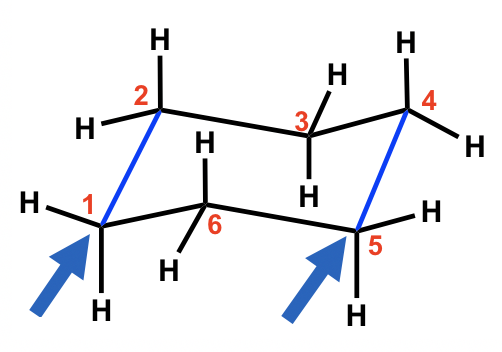
How do I rotate the ring inversion chair. Chair conformations are present. Ask Question Asked 5 years 6 months ago. Bruice Organic Chemistry 6th Edition Chapters 21-215 33-35 51-58 511-513 517 520-5. Chem 201Beauchamp Topic 6 Conformations cyclohexanes 2 How to draw a Newman projection of a cyclohexane ring. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
It 4762 kcalmol1 Similarly most mono- and di-substituded is likely that intramolecular hydrogen bonding is not contribut- cyclohexanes exist preferentially in the chair conformation with ing to the preference for chair conformers with axial substituents equatorial substituents12 However for molecules in which large as the crystal. Chair conformation lounge chair - used to kick back and relax. Draw the second chair conformation ring-flip-check this post if not sure. Viewed 2k times 2 begingroup I have two questions regarding this solution. Cyclohexane is the chair conformation shown below. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
Active 5 years 6 months ago. Cyclohexane is the chair conformation shown below. Chair conformation lounge chair - used to kick back and relax. Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. 3 13 Solutions To Chapter 3 Exercises Chemistry Libretexts.

The lactam ring adopts an envelope conformation whereas the cyclohexane ring has an almost ideal chair conformation. Chair conformations are present. 418 YOUR TURN Draw the chair conformation of cyclohexane in Figure 4-28 using the steps. When the chair is flipped the axial positions become equatorial and vice versa. Active 5 years 6 months ago. Black Outdoor Balcony Bar Set Zulily Diseno Pequeno Balcon Muebles De Terraza Pequena Diseno De Balcon.

To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Now that the molecule is in the boat conformation the energy can be determined for a boat conformation. A flip-chair conformation is the conversion of one cyclohexane chair conformation to another by rotation around the carbon-carbon single bonds. Finally add an upward-pointing V tip to the other end this is the nose of the chair. If you adjust the t-butyl group on the 4 th carbon by dragging the carbon upwards you can now transform the molecule into a boat conformation as seen in Figure 3 below. Levels Of Mastery Master Organic Chemistry.
Chair conformation lounge chair - used to kick back and relax. Observe if the puckered ring oxygen atom lies above p ve the plane of the ring or below p -ve. Chair conformations are present. Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation. And now the stabilities. 4 3 Conformation Analysis Of Cyclohexane Organic Chemistry.

The steps involved in drawing the chair conformation of cyclohexane. 418 YOUR TURN Draw the chair conformation of cyclohexane in Figure 4-28 using the steps. Finally add an upward-pointing V tip to the other end this is the nose of the chair. Another conformation which is important in any conformational analysis is the transition state or maximum energy conformation on the rotational pathFor cyclohexane this is the so-called half-chair conformation in which now 5 carbons are co-planar and only one is puckered out of the plane. An examination of puckering parameters torsion angles and model Dreiding of the title compounds clearly indicates that the ring A has slightly distorted chair conformation and ring B has slightly distorted envelope conformation. Cyclohexane Chair Conformation Stability Which One Is Lower Energy.
A molecule might exist with either the S or R configuration at a chirality center but these two possibilities represent different moleculesthey cannot interconvert without breaking and reforming chemical bonds. Ask Question Asked 5 years 6 months ago. First thing is to number your substituents or carbon atom on the initial molecule like you did already. Stretched-Chair Conformation Figures 1 and 2 were the energies determined for the chair conformation. As we learned in Chapter 3 configuration relates to the connectivity of atoms. 4 4 Substituted Cyclohexanes Organic Chemistry.
Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation. A flip-chair conformation is the conversion of one cyclohexane chair conformation to another by rotation around the carbon-carbon single bonds. When the chair is flipped the axial positions become equatorial and vice versa. The following guidelines can be used to determine the percent distribution of two chair conformers using the Gibbs free energy equation and some simple algebra. The free energy ΔG of the equilibrium process going from one conformer to the other ie from A to B as shown in step 4 or 5 can be calculated from the equation below where the. 4 3 Conformation Analysis Of Cyclohexane Organic Chemistry.

The bromine is in the same configuration in compound III and ring A also adopts the chair conformation. Next add a downward-pointing V tip to one end this is the tail of the chair. To begin start by drawing two lines that are parallel to each other but not perfectly horizontal as shown here. Draw the second chair conformation ring-flip-check this post if not sure. Both compounds adopt chairenvelope conformation. Small Spaces Usual Tend To Pressure Us And Pose Immense Difficulties Into Its Design And Co Small Balcony Design Balcony Furniture Apartment Balcony Decorating.

17 Stereochemistry Conformation and Configuration Reference. The steps involved in drawing the chair conformation of cyclohexane. There are four molecules in the asymmetric unit of γ-spirolactam 1a which show small conformational differences. 418 YOUR TURN Draw the chair conformation of cyclohexane in Figure 4-28 using the steps. Stretched-Chair Conformation Figures 1 and 2 were the energies determined for the chair conformation. Ring Flipping An Overview Sciencedirect Topics.
An examination of puckering parameters torsion angles and model Dreiding of the title compounds clearly indicates that the ring A has slightly distorted chair conformation and ring B has slightly distorted envelope conformation. If there are no substituents nonhydrogen atoms or groups attached to the ring the two forms will have equal energy. As we learned in Chapter 3 configuration relates to the connectivity of atoms. Chair conformations are present. Now that the molecule is in the boat conformation the energy can be determined for a boat conformation. The Cyclohexane Chair Flip Master Organic Chemistry.

If there are no substituents nonhydrogen atoms or groups attached to the ring the two forms will have equal energy. The relative stability of substituents is determined by repulsive non- bonded interactions steric compression. An examination of puckering parameters torsion angles and model Dreiding of the title compounds clearly indicates that the ring A has slightly distorted chair conformation and ring B has slightly distorted envelope conformation. Here we have one chair conformation of methylcyclohexane and this is carbon one you can see we have a methyl group that is axial up at carbon one we also have a hydrogen and Ive made the hydrogen green so we can tell apart from the other hydrogens and this hydrogen is equatorial down at carbon one now this chair conformation is in equilibrium with another chair conformation and we can get to the other chair conformation. In II ring A has a chair conformation with a trans AB ring fusion. Chair Conformations Of Glucose Youtube.
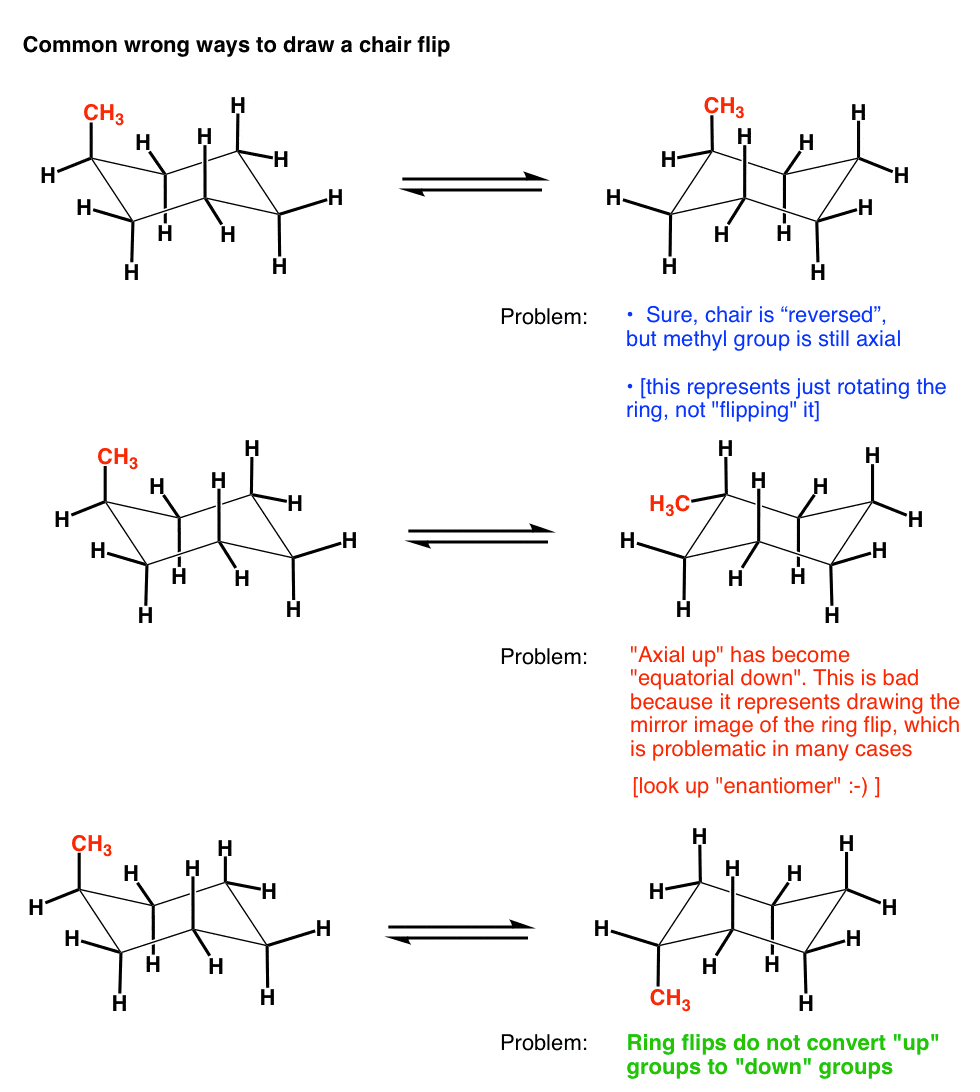
Chair conformation is the term used in organic chemistry that represents the chair-like structure of a carbon ring consisting of six carbon atoms. How do I rotate the ring inversion chair. I cant see how the structure drawn is the enantiomer. And now the stabilities. The following chair conformation of cyclohexane is obtained after the one in Figure 4-28 has undergone a chair flip. The Cyclohexane Chair Flip Master Organic Chemistry.