Expansions and renovations to existing biological facilities and construction of new facilities provide a unique opportunity to rethink basic design strategies and use new technologies to build a better facility that will improve compliance. We can help you with the creation of a facility concept and quickly find answers to fundamental questions in order to meet demanding timelines. bioprocess facility design.
Bioprocess Facility Design, Facilities Design Introduction 111. John Wiley Sons Jan 26 2018 - Science - 384 pages. This module focuses on how to take a process from a paper design to fully-operational facility and will enable you to.
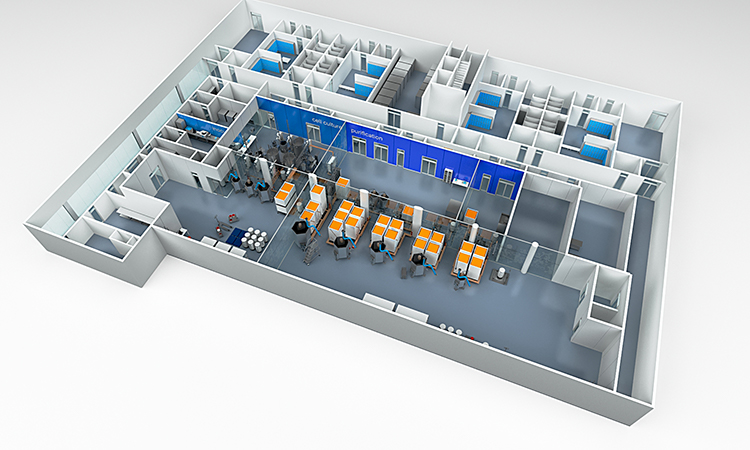 Biopharma Facility Design Lessons Learned On The Dance Floor Pharmaceutical Engineering From ispe.org
Biopharma Facility Design Lessons Learned On The Dance Floor Pharmaceutical Engineering From ispe.org
Success factors Integrate early on RD Engineering and Manufacturing Teams Assess each Bioprocess option from an Integrated industrial view Select optimal option based on Scale-up reliability economics Use Modeling to evaluate each Bioprocess impact on Facility Design Utilities Costs. In other words facilities design attempts to organize the tangible fixed assets of an. We bring our clients practical expertise on all biotechnology projects.
MAb Direct Fixed Capital DFC 1657 Million Insurance 0015 25 Local Taxes 0025 41 DFC Contractors Fee 0055 91 Maintenance 006 99 Depreciation period 10 years Depreciation 010 TCI 166 Annual Facility-Dependent Cost 423 Million.
Facilities Design Introduction 111. This often means developing single-use SU or hybrid bioprocessing facilities with reusable legacy equipment for production of monoclonal antibodies MAbs antibodydrug conjugates ADCs and viral-based vectors. Facilities Design Definition Facilities Design is the planning and design of the physical environment of an activity to best support the execution of this activity. Design a plant layout considering the relevant regulatory requirements. Essential information for architects designers engineers equipment suppliers and other professionals who are working in or entering the biopharmaceutical manufacturing field. Bioprocess Facility Design Layout Rules And Configurations Jul 28 2016 As we discussed in our previous article there are multiple variables that must be considered in the layout of a biopharmaceutical facility and many of these variables are interdependent.
Another Article :
We know the bioprocessing industry and the needs of developers. Design a plant layout considering the relevant regulatory requirements. Impact on facility design cost of goods and cost of development for monoclonal antibodies Biotechnol Bioeng. Unlock the potential of your biomass feedstock convert your feedstock to the required product andor kickstart the. Facility Design Solutions Genderless AseptiQuik L Series Connectors 5152018 Genderless AseptiQuik Large Format 34 1 and 1½ sanitary Connectors enable quick and easy sterile connections even in non-sterile environments. 2.

For many companies facility design begins by engaging an engineering consultancy to develop a layout that maximizes space use. We know the bioprocessing industry and the needs of developers. Facility and Process Design. This module focuses on how to take a process from a paper design to fully-operational facility and will enable you to. Facilities Design Introduction 199 Chapter 11. Biosimilar Development Layout Design Development Layout.

We know the bioprocessing industry and the needs of developers. The ballroom concept was originally defined in the International Society of Pharmaceutical Engineers ISPE Baseline Guide. Facilities Design Introduction 199 Chapter 11. Facilities Design Introduction 111. Design a plant layout considering the relevant regulatory requirements. Pdf Concept Facility Based On Single Use Systems Part 2 Leading The Way For Biomanufacturing In The 21st Century Semantic Scholar.

This often means developing single-use SU or hybrid bioprocessing facilities with reusable legacy equipment for production of monoclonal antibodies MAbs antibodydrug conjugates ADCs and viral-based vectors. OFFERING BIOPROCESS EXPERTISE TO THE BIOPHARMACEUTICAL INDUSTRY. Key Drivers and Decisions The Oxbox facility design was adapted from a large existing building that originally had been constructed and used as a postal sorting office. Essential information for architects designers engineers equipment suppliers and other professionals who are working in or entering the biopharmaceutical manufacturing field. The Bioprocess Pilot Facility BV. Biotech Industry S Quest For Optimized Manufacturing Facility Design Pharmaceutical Engineering.

Jeffery Odum Michael C. We know the bioprocessing industry and the needs of developers. We are experienced in biofuels and process development and design. Process Architecture in Biomanufacturing Facility Design. Design a plant layout considering the relevant regulatory requirements. Efficient Flexible Facilities For The 21st Century Bioprocess Internationalbioprocess International.

This webinar will discuss how process and regulations are changing the modern biotechnology facility and process design. Volume 6 Biopharmaceutical Manufacturing Facilities 2013 as A large manufacturing area that has. John Wiley Sons Jan 26 2018 - Science - 384 pages. We can help you with the creation of a facility concept and quickly find answers to fundamental questions in order to meet demanding timelines. A case study is presented describing initiatives and bioprocessing approaches to maximize capacity utilization within a new large scale 12x 15kL drug substance biologics manufacturing facility at Samsung Biologics. Facility Design For Biomanufacturing Operations Bioprocess Internationalbioprocess International.

Design a plant layout considering the relevant regulatory requirements. MAb Direct Fixed Capital DFC 1657 Million Insurance 0015 25 Local Taxes 0025 41 DFC Contractors Fee 0055 91 Maintenance 006 99 Depreciation period 10 years Depreciation 010 TCI 166 Annual Facility-Dependent Cost 423 Million. The 7800-m 2 open-plan facility has very few supporting pillars and a significant height 9 m to the eaves. Process Architecture in Biomanufacturing Facility Design. This webinar will discuss how process and regulations are changing the modern biotechnology facility and process design. Integrated Process Modelling Very Useful Bioprocess Digital Twin Pharmaceutical Engineering.

The ballroom concept includes placing the bioprocess equipment on wheels rather than in permanently fixed positions. Successfully meeting demand for both productivity and cost control requires simultaneously addressing performance and efficiency in your specific. Process Architecture in Biomanufacturing Facility Design. Facility design manufacturing and validation strategies utilizing two harvest and purification suites with regards to cadence and product changeover PCO will. For many companies facility design begins by engaging an engineering consultancy to develop a layout that maximizes space use. Facility Design For Continuous Bioprocessing And Smart Manufacturing Attributes For Success American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology.

Unlock the potential of your biomass feedstock convert your feedstock to the required product andor kickstart the. Bioprocess Facility Design Layout Rules And Configurations Jul 28 2016 As we discussed in our previous article there are multiple variables that must be considered in the layout of a biopharmaceutical facility and many of these variables are interdependent. The Bioprocess Pilot Facility BV. Facility Design Solutions Genderless AseptiQuik L Series Connectors 5152018 Genderless AseptiQuik Large Format 34 1 and 1½ sanitary Connectors enable quick and easy sterile connections even in non-sterile environments. Since 2011 we have had a national presence in offering quality compliance positioning process control strategies and cGMP facility designstart-up support to our biopharmaceutical clients. Cell And Gene Therapy Manufacturing Facilities Bioprocess Internationalbioprocess International.

Facility Design andor Selection With process development near completion at the pilot plant level simulation tools are used to systematically. Jeffery Odum Michael C. Impact on facility design cost of goods and cost of development for monoclonal antibodies Biotechnol Bioeng. Facility Design andor Selection With process development near completion at the pilot plant level simulation tools are used to systematically. Facilities Design Introduction 199 Chapter 11. Ptt 402 Biotechnology Facility Design Lecture 4 Instrumentation.

OFFERING BIOPROCESS EXPERTISE TO THE BIOPHARMACEUTICAL INDUSTRY. Design a plant layout considering the relevant regulatory requirements. The ballroom concept includes placing the bioprocess equipment on wheels rather than in permanently fixed positions. Jeffery Odum Michael C. We bring our clients practical expertise on all biotechnology projects. Bioprocess Plant Design And Economic Analysis Of An Environmentally Friendly Insect Controller Agent Produced With Azadirachta Indica Cell Culture Sciencedirect.

Focusing on how to take a process from a paper design to a fully-operational facility. Jeffery Odum Michael C. Merrick is a leader in developing and commercializing bioprocessing facilities. In other words facilities design attempts to organize the tangible fixed assets of an. This module focuses on how to take a process from a paper design to fully-operational facility and will enable you to. Ge Modular Production Facilities The Future Of All Biologics.

Successfully meeting demand for both productivity and cost control requires simultaneously addressing performance and efficiency in your specific. This module focuses on how to take a process from a paper design to fully-operational facility and will enable you to. Facilities Design Definition Facilities Design is the planning and design of the physical environment of an activity to best support the execution of this activity. Essential information for architects designers engineers equipment suppliers and other professionals who are working in or entering the biopharmaceutical manufacturing field. Focusing on how to take a process from a paper design to a fully-operational facility. Building The Ideal Bioprocessing Strategy.

Since 2011 we have been working with our clients nationwide by offering them top-notch. Facility Design Solutions Genderless AseptiQuik L Series Connectors 5152018 Genderless AseptiQuik Large Format 34 1 and 1½ sanitary Connectors enable quick and easy sterile connections even in non-sterile environments. Expansions and renovations to existing biological facilities and construction of new facilities provide a unique opportunity to rethink basic design strategies and use new technologies to build a better facility that will reduce costs and improve compliance. Merrick is a leader in developing and commercializing bioprocessing facilities. Single-use systems drive modular facilities Evolving regulatory environment biologics landscape Process efciencies intensify Biologics are complex and every process is unique. Facility Design For Continuous Bioprocessing And Smart Manufacturing Attributes For Success American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology.

Expansions and renovations to existing biological facilities and construction of new facilities provide a unique opportunity to rethink basic design strategies and use new technologies to build a better facility that will improve compliance. Process Architecture in Biomanufacturing Facility Design. Design a plant layout considering the relevant regulatory requirements. Facility Design Solutions Genderless AseptiQuik L Series Connectors 5152018 Genderless AseptiQuik Large Format 34 1 and 1½ sanitary Connectors enable quick and easy sterile connections even in non-sterile environments. Since 2011 we have been working with our clients nationwide by offering them top-notch. Designing Bioprocess Systems For Modular Needs.









