The patient will be beneficial from the study. 11 Bioequivalence experimental study designs 1Completely randomized designs. bioequivalence study design ppt.
Bioequivalence Study Design Ppt, Read together with Appendix IV. Reflects better therapeutic efficacy of a drug. Presents the recent developments in methodology including population and individual bioequivalence.
 Ppt Statistical Considerations Powerpoint Presentation Free Download Id 805331 From slideserve.com
Ppt Statistical Considerations Powerpoint Presentation Free Download Id 805331 From slideserve.com
Consists of both single dose multiple dose. 5 132 Single- vs. Provides a practical overview of the design and analysis of bioequivalence studies.
11 Bioequivalence experimental study designs 1Completely randomized designs.
8162006 120000 AM Document presentation format. 5 131 Crossover design and alternatives. Open Access International Journal of. And then repeat for all other treatments. The basic assumption The basic assumption underlying the bioequivalence concept is that similar plasma time courses lead to essentially the same effects pharmacological toxic therapeutic Classical objections Plasma concentration is not biophase concentration there is no univocal relationships between. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author.
Another Article :

Make a new Worksheet with the right headers for your bioequivalence data. Objective The basic design for bioequivalence study is determined by. Choose Insert NCA and Toolbox Bioequivalence which will open a. Copy-paste the data into the worksheet 3. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author. Ppt Statistical Considerations Powerpoint Presentation Free Download Id 805331.

Design and Analysis of Pharmacodynamic Crossover Studies Conducted to Establish Bioequivalence of Inhaled Corticosteroids Author. Pk and Pd of drug substance 5. Design and Analysis of Pharmacodynamic Crossover Studies Conducted to Establish Bioequivalence of Inhaled Corticosteroids Author. Read together with Appendix IV. 5 131 Crossover design and alternatives. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.

Pk and Pd of drug substance 5. Drug absorption pattern in disease states can be evaluated. 90 CI of mean TR. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in. Ppt Seminar On Crossover Design Powerpoint Presentation Free Download Id 3801391.

Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in. Consists of both single dose multiple dose. Bioequivalence BE studies investigate and compare the pharmacologic characteristics of different formulations of a drug product with respect to the rate and extent of exposure to a new formulation Test and a reference listed. Before a clinical trial is conducted design of study has to be selected. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. Ppt Seminar On Crossover Design Powerpoint Presentation Free Download Id 3801391.
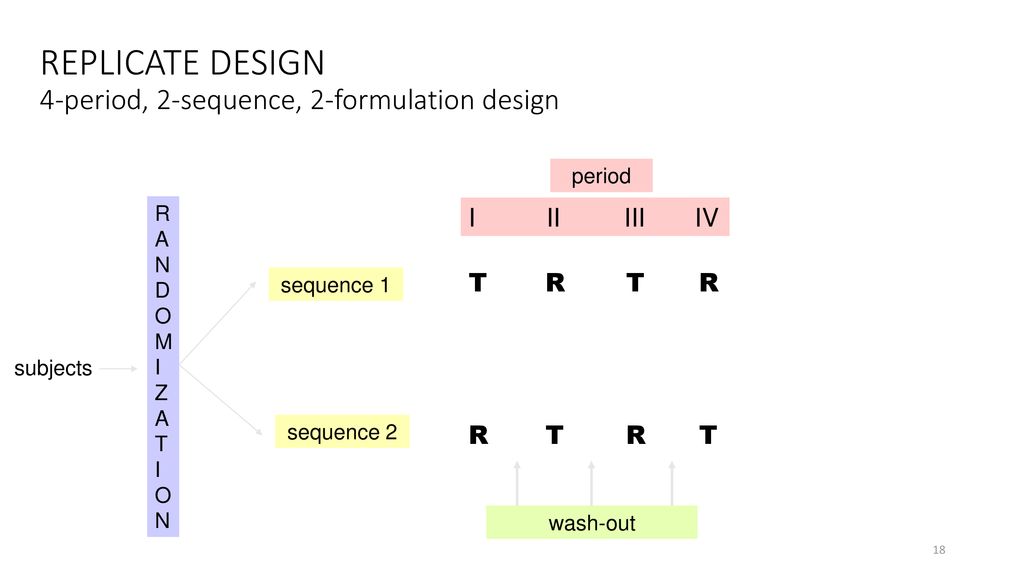
Pharmaceutically equivalent drug in the same dose strength in similar dosage forms eg immediate release or controlled release and given by the same route of administration. And then repeat for all other treatments. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. International Journal of BioAnalytical Methods BioEquivalence Studies ISSN 2470-4490 - International Journal of BioAnalytical Methods BioEquivalence Studies IJBMBS ISSN 2470-4490 is a peer-reviewed open access journal wherein publication related to high-impact research articles contributes on the fundamental and applied topics of analytical and bioanalytical science including chromatography. Absolute and Relative bioavailabilty are discussed. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
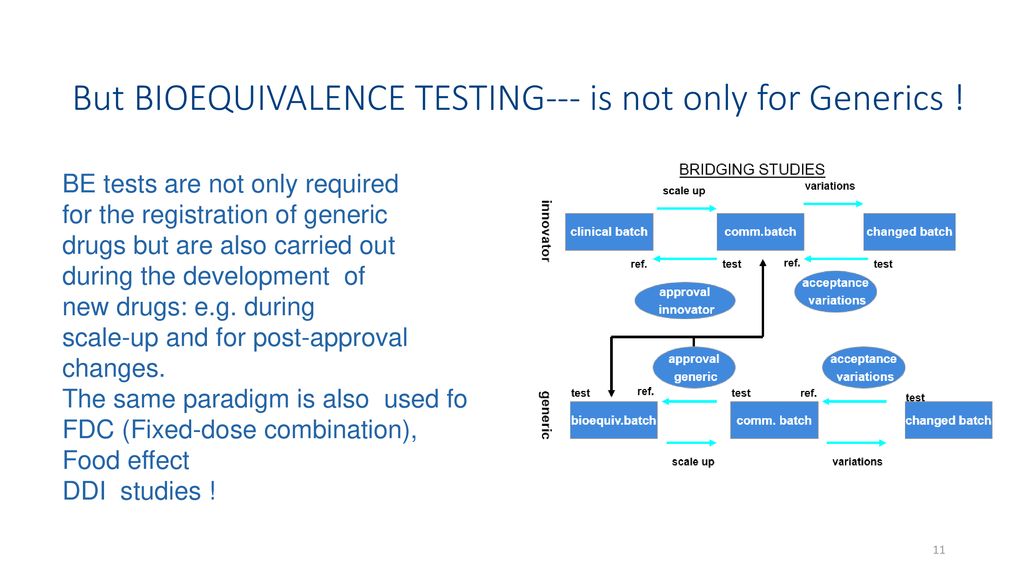
Any design that can remove this variation from the comparison in average bioavailability between formulations would be the appropriate one. Study report synopses for bioequivalence or comparative bioavailability studies conducted during formulation development should also be included in Module 27. Bioequivalence BE studies investigate and compare the pharmacologic characteristics of different formulations of a drug product with respect to the rate and extent of exposure to a new formulation Test and a reference listed. Copy-paste the data into the worksheet 3. Before a clinical trial is conducted design of study has to be selected. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
Provides a practical overview of the design and analysis of bioequivalence studies. Open Access International Journal of. The availability of analytical methods 4. DESIGN OF BIOEQUIVALENCE STUDIES The test and reference drug formulations must contain. Copy-paste the data into the worksheet 3. Bioequivalence.

A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. On-screen Show 43 Other titles. Pk and Pd of drug substance 5. Bioequivalence Study Reporting Format 1. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.
Bioequivalence studies comparing the product applied for with non-EU reference products should not be submitted and do not need to be included in the list of studies. Activate the project by clicking its name in the Object Browser left side panel. Bioequivalence studies comparing the product applied for with non-EU reference products should not be submitted and do not need to be included in the list of studies. Pharmacokinetics and Pharmacodynamics of the study designs make an important role. Presents the recent developments in methodology including population and individual bioequivalence. Bioequivalence.
It includes randomized two-period two-sequence single dose cross-over design parallel design and replicate designs. Study report synopses for bioequivalence or comparative bioavailability studies conducted during formulation development should also be included in Module 27. Read together with Appendix IV. 5 131 Crossover design and alternatives. Bioequivalence BE studies investigate and compare the pharmacologic characteristics of different formulations of a drug product with respect to the rate and extent of exposure to a new formulation Test and a reference listed. Bioequivalence.

Bioavailability and Boequivalenceppt - Free download as Powerpoint Presentation ppt PDF File pdf Text File txt or view presentation slides online. Before a clinical trial is conducted design of study has to be selected. Title Page 11 Study Title 12 Name and address of Sponsor 13 Name person in charge and address of Institution 14 Name and address of Principal Investigator. 13 Design and conduct of bioequivalence studies. On-screen Show 43 Other titles. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.

On-screen Show 43 Other titles. The basic assumption The basic assumption underlying the bioequivalence concept is that similar plasma time courses lead to essentially the same effects pharmacological toxic therapeutic Classical objections Plasma concentration is not biophase concentration there is no univocal relationships between. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in. Bioavailability and Boequivalenceppt - Free download as Powerpoint Presentation ppt PDF File pdf Text File txt or view presentation slides online. 11 Bioequivalence experimental study designs 1Completely randomized designs. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.

13 Design and conduct of bioequivalence studies. Presents the recent developments in methodology including population and individual bioequivalence. Open Access International Journal of. The patient will be beneficial from the study. If there are 20 subjects number the from 1 to 20. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
The patient will be beneficial from the study. Drug absorption pattern in disease states can be evaluated. Randomly select non repeating numbers among these labels for the first treatment. Consists of both single dose multiple dose. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.
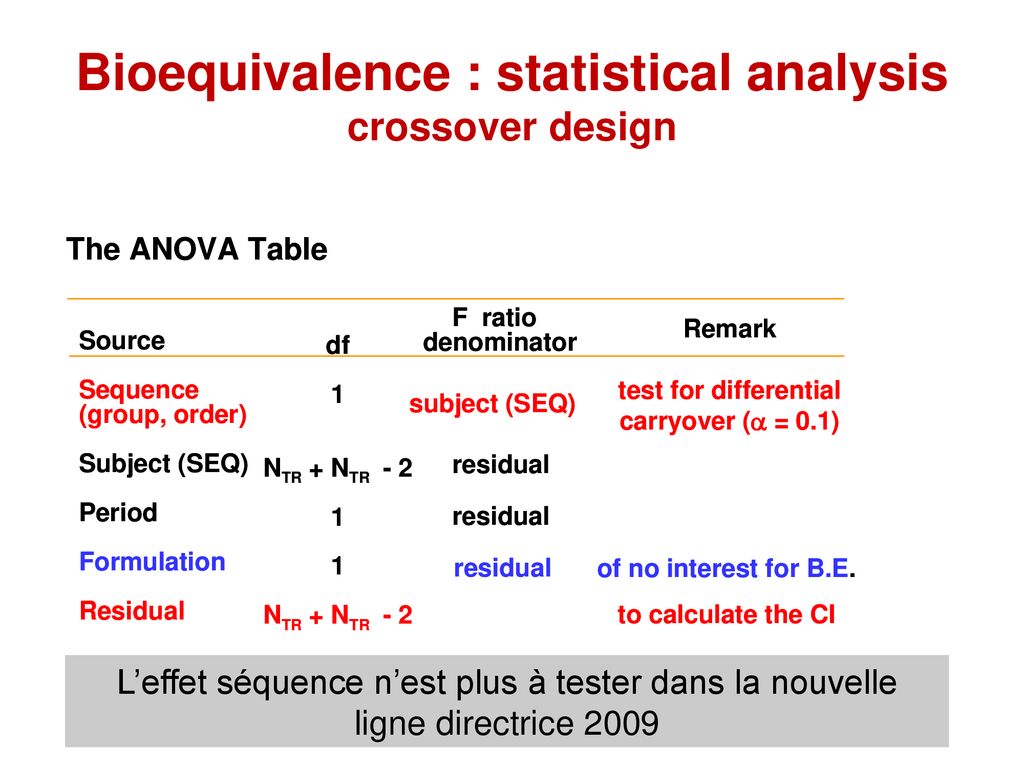
Open Access International Journal of. On-screen Show 43 Other titles. Criterion for choosing an appropriate design is whether or not the selected design can identify estimate and isolate the intersubject variability. Activate the project by clicking its name in the Object Browser left side panel. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.









