Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. Comparison of product-specific recommendations of EMA and US-FDA 53. bioequivalence study design fda.
Bioequivalence Study Design Fda, Example for bioequivalence 6 Figure 4. 81 Clinical Study Design Study Design crossover parallel Fed Fasted Inclusion Exclusion Restriction Standardization of Study Condition Drug Administration Removal of Subject from Assessment Health Screening. 90 CI of mean TR.
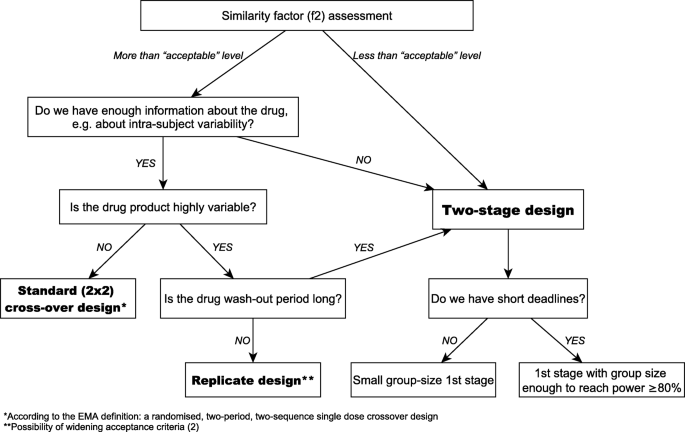 10th Anniversary Of A Two Stage Design In Bioequivalence Why Has It Still Not Been Implemented Springerlink From link.springer.com
10th Anniversary Of A Two Stage Design In Bioequivalence Why Has It Still Not Been Implemented Springerlink From link.springer.com
The Reference Listed Drug. Example for bioequivalence 6 Figure 4. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current.
Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current.
Show in their Journal of BE BA article El-Tahtawy A Harrison F Zirkelbach JF Jackson AJ 2011 Bioequivalence of Long Half-Life Drugs Informative Sampling Determination Parallel Designed Studies. If the product is intended for use in both sexes inclusion of similar proportions of males and females should be intended. This 42page guidance supersedes the December 2013 draft guidance of the. In 1984 the United States Food and Drug Administration FDA was authorized to approve generic drug products under the Drug Price Competition and Patent Term Restoration Act based on evidence of average bioequivalence in drug absorption through the conduct of bioavailability and bioequivalence studies. Bioequivalence study provides bridging of the full clinical dataset held by Medsafe for the innovator medicine to support the efficacy and safety of generic medicines entering the New Zealand market. It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests.
Another Article :

Read together with Appendix IV. By Bob Pollock Aug 20 2021 Bioequivalence FDA Generics PSGs Regulatory Affairs. 28 rows The selection of the method used to demonstrate bioequivalence depends upon the. On January 15 2021 the FDA issued the guidance titled Protecting Participants in Bioequivalence Studies for Abbreviated New Drug Applications During the COVID-19 Public Health Emergency. Comparison of product-specific recommendations of EMA and US-FDA 53. Bioequivalence Studies A Statistical Approach Through R.

90 CI of mean TR. Randomized two-period two-sequence Crossover design with adequate washout period If the. According to the current FDA guidance in vivo bioequivalence studies should be conducted in individuals 18 years or older who are representative of the general population taking into account for age sex and race. All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. Approaches To Supply Bioequivalent Oral Solid Pharmaceutical Formulations Through The Lifecycles Of Products Four Media Dissolution Monitoring Program In Japan Sciencedirect.

90 CI of mean TR. When comparing three drug products researchers could use a 63 six-sequence three-period Williams design and for comparing four drug products they would use a 44 four-sequence four-period Williams design. Bioequivalence BE studies are performed based on the requirements set forth in part 320 of section 21 of the Code of Federal Regulations CFR and guidance given by the US Food and Drug Administrations FDAs Center for Drug Evaluation and Research CDER 1. This study design enabled us to determine the true intrasubject variability for the test and reference products independently and enabled us to apply the scaled-average-bioequivalence approach which offers more-flexible bioequivalence acceptance criteria for highly variable drug products according to the current EMACHMP Guidelines on the Investigation of. This month FDA issued a new guidance for industry concerning the submission of summary bioequivalence data for abbreviated new drug applications ANDAs. Statistical Evaluation Of Bioequivalence Studies Bebac.

FDA Issues Guidance on Bioequivalence Studies. This month FDA issued a new guidance for industry concerning the submission of summary bioequivalence data for abbreviated new drug applications ANDAs. It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. Randomized two-period two-sequence Crossover design with adequate washout period If the. 90 CI of mean TR. Fda Advisory Committee Discussion And Guidance On Recent Bioequivalence Download Table.

It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. Today the FDA announced the issuance of a revised draft guidance titled Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry here. The US Food and Drug Administration FDA has released new guidance on the agencys compliance policy regarding samples used in bioavailability and bioequivalence studies. It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. If the product is intended for use in both sexes inclusion of similar proportions of males and females should be intended. Pdf The Basic Regulatory Considerations And Prospects For Conducting Bioavailability Bioequivalence Ba Be Studies An Overview Semantic Scholar.
In 1984 the United States Food and Drug Administration FDA was authorized to approve generic drug products under the Drug Price Competition and Patent Term Restoration Act based on evidence of average bioequivalence in drug absorption through the conduct of bioavailability and bioequivalence studies. The bioequivalence study uses anappropriate statistical assessment to determine whether. 81 Clinical Study Design Study Design crossover parallel Fed Fasted Inclusion Exclusion Restriction Standardization of Study Condition Drug Administration Removal of Subject from Assessment Health Screening. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in vitro dissolution generics. Microsoft Word - Bioequivalence Study Reporting Format. Pharmaceuticals Free Full Text Model Based Approach For Designing An Efficient Bioequivalence Study For Highly Variable Drugs Html.

Read together with Appendix IV. Show in their Journal of BE BA article El-Tahtawy A Harrison F Zirkelbach JF Jackson AJ 2011 Bioequivalence of Long Half-Life Drugs Informative Sampling Determination Parallel Designed Studies. Question to be answered nature of reference drug dosage form benefit-risk ratio As far as possible the study should be of crossover design suitably randomized Ideal design. Comparison of product-specific recommendations of EMA and US-FDA 53. AUC after single-dose administration and at steady-state 8 List of Tables Table 1. Considerations For Planning And Designing A Bioequivalence Be.

All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. According to the current FDA guidance in vivo bioequivalence studies should be conducted in individuals 18 years or older who are representative of the general population taking into account for age sex and race. By Bob Pollock Aug 20 2021 Bioequivalence FDA Generics PSGs Regulatory Affairs. This guidance provides recommendations to study sponsors for the continuation or initiation of their bioequivalence BE studies during this COVID-19 public. Show in their Journal of BE BA article El-Tahtawy A Harrison F Zirkelbach JF Jackson AJ 2011 Bioequivalence of Long Half-Life Drugs Informative Sampling Determination Parallel Designed Studies. Average Population And Individual Bioequivalence Semantic Scholar.

Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. Today the FDA announced the issuance of a revised draft guidance titled Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry here. 81 Clinical Study Design Study Design crossover parallel Fed Fasted Inclusion Exclusion Restriction Standardization of Study Condition Drug Administration Removal of Subject from Assessment Health Screening. 41 Design conduct and evaluation of bioequivalence studies The number of studies and study design depend on the physico-chemical characteristics of the substance its pharmacokinetic properties and proportionality in composition and should be justified. It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. Pdf Bioavailability And Bioequivalence An Fda Regulatory Overview.

An applicant may request a waiver of in vivo bioequivalence testing for the 20 mg strength provided that it 1 submits. All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. Read together with Appendix IV. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in vitro dissolution generics. Avoiding Risky Business Developing Effective Rems Plans Http Whybenchmarking Com 2013 07 05 Avoiding Risky Business Risky Business How To Plan Development.

If the product is intended for use in both sexes inclusion of similar proportions of males and females should be intended. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. This document specifies the requirements for the design conduct and evaluation of bioequivalence studies for immediate release dosage forms with systemic action. This month FDA issued a new guidance for industry concerning the submission of summary bioequivalence data for abbreviated new drug applications ANDAs. The Reference Listed Drug. Percent Of Studies Passing Bioequivalence Be Power Curves Average Download Scientific Diagram.

In 1984 the United States Food and Drug Administration FDA was authorized to approve generic drug products under the Drug Price Competition and Patent Term Restoration Act based on evidence of average bioequivalence in drug absorption through the conduct of bioavailability and bioequivalence studies. The US Food and Drug Administration FDA has released new guidance on the agencys compliance policy regarding samples used in bioavailability and bioequivalence studies. All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. The Reference Listed Drug. 90 CI of mean TR. The Impact Of New Partial Auc Parameters For Evaluating The Bioequivalence Of Prolonged Release Formulations Sciencedirect.

90 CI of mean TR. This 42page guidance supersedes the December 2013 draft guidance of the. 81 Clinical Study Design Study Design crossover parallel Fed Fasted Inclusion Exclusion Restriction Standardization of Study Condition Drug Administration Removal of Subject from Assessment Health Screening. This document specifies the requirements for the design conduct and evaluation of bioequivalence studies for immediate release dosage forms with systemic action. All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. 10th Anniversary Of A Two Stage Design In Bioequivalence Why Has It Still Not Been Implemented Springerlink.

The guidance is meant to clarify the requirements for the submission of bioequivalence data that were published in 2009 1. The FDA received a few comments related to truncation. Show in their Journal of BE BA article El-Tahtawy A Harrison F Zirkelbach JF Jackson AJ 2011 Bioequivalence of Long Half-Life Drugs Informative Sampling Determination Parallel Designed Studies. Today the FDA announced the issuance of a revised draft guidance titled Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry here. All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. A Visual Representation Of Some Possible Results Of The Statistical Download Scientific Diagram.

An applicant may request a waiver of in vivo bioequivalence testing for the 20 mg strength provided that it 1 submits. This study design enabled us to determine the true intrasubject variability for the test and reference products independently and enabled us to apply the scaled-average-bioequivalence approach which offers more-flexible bioequivalence acceptance criteria for highly variable drug products according to the current EMACHMP Guidelines on the Investigation of. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in vitro dissolution generics. What is the justification for this. Microsoft Word - Bioequivalence Study Reporting Format. Establishing Virtual Bioequivalence And Clinically Relevant Specifications Using In Vitro Biorelevant Dissolution Testing And Physiologically Based Population Pharmacokinetic Modeling Case Example Naproxen Sciencedirect.









