The guidance describes important principles for designing conducting and reporting the results from an adaptive clinical trial. The US Food and Drug Administration FDA last week finalized guidance on adaptive clinical trial designs for drugs and biologicsThis document provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics licensing applications BLAs or supplemental applications on. adaptive design clinical trials for drugs and biologics.
Adaptive Design Clinical Trials For Drugs And Biologics, This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. FDA issues guidance for industry on adaptive designs for clinical trials of drugs and biologics. The September 2018 release replaces the 2010 draft guidance issued by the FDA.
 Have Clinical Trials In Hiv Finally Matured The Lancet Hiv From thelancet.com
Have Clinical Trials In Hiv Finally Matured The Lancet Hiv From thelancet.com
For the purposes of this guidance an adaptive design is defined as a clinical trial design that 44 allows for prospectively planned modifications to one or more aspects of the design based on. On November 29 the Food and Drug Administration FDA issued a final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. Adaptive designs for exploratory clinical trials deal mainly with.
The purpose is to.
The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. Today the Food and Drug Administration FDA issued final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry December 2019 Download the Final Guidance Document Read the Federal Register Notice Final Level 1 Guidance. The guidance provides information to. The US Food and Drug Administration FDA last week finalized guidance on adaptive clinical trial designs for drugs and biologicsThis document provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics licensing applications BLAs or supplemental applications on. By casting dose finding as a Bayesian model selection problem we propose an adaptive design by simultaneously incorporating the toxicity and efficacy outcomes to select the optimal biological dose OBD in phase III clinical trials.
Another Article :

This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Additional copies are available from. Adaptive designs for exploratory clinical trials deal mainly with. The clinical trial landscape has changed since 2010 regards the FDAs stance on. The September 2018 release replaces the 2010 draft guidance issued by the FDA. Clinical Trial Designs Basket Umbrella Platform Trial Designs Part Ii Credevo Articles.
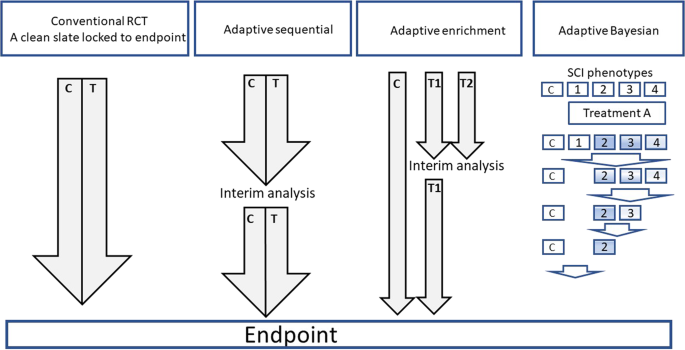
An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. The September 2018 release replaces the 2010 draft guidance issued by the FDA. Adaptive Trial Designs For Spinal Cord Injury Clinical Trials Directed To The Central Nervous System Spinal Cord.
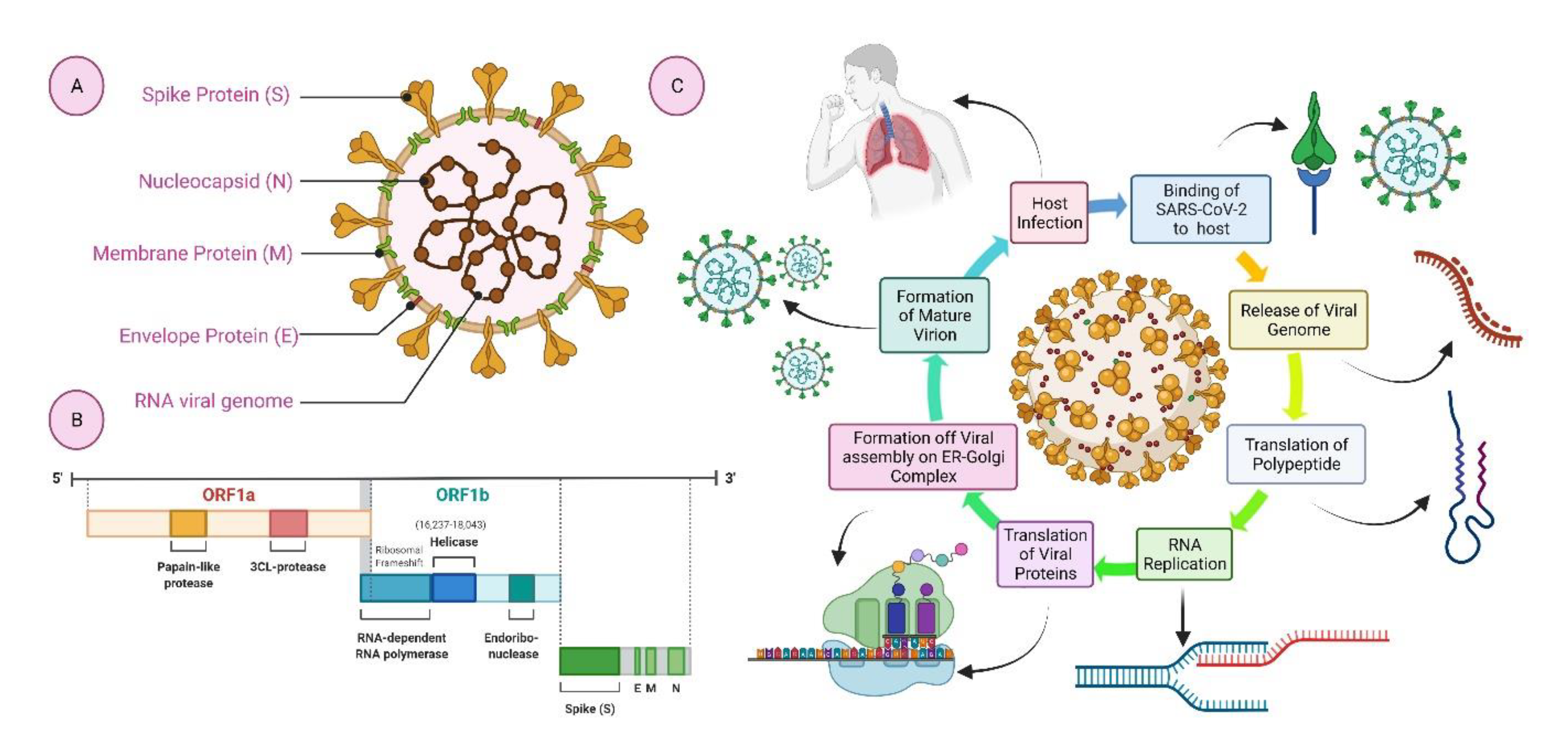
Adaptive Design Clinical Trials for Drugs and Biologics which defines adaptive designs as studies that include a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data. This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs. The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Additional copies are available from. Biologics Free Full Text Nucleic Acid Vaccines For Covid 19 A Paradigm Shift In The Vaccine Development Arena Html.

An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Today the Food and Drug Administration FDA issued final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry December 2019 Download the Final Guidance Document Read the Federal Register Notice Final Level 1 Guidance. The US Food and Drug Administration FDA last week finalized guidance on adaptive clinical trial designs for drugs and biologicsThis document provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics licensing applications BLAs or supplemental applications on. The Guidance will help drug sponsors run clinical trials more efficiently and effectively increasing their chances of having a successful application to market a new drug or biologic product. Clinical Trial Design Past Present And Future In The Context Of Big Data And Precision Medicine Li 2020 Cancer Wiley Online Library.

This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. The clinical trial landscape has changed since 2010 regards the FDAs stance on. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. The purpose is to. An Overview Of Platform Trials With A Checklist For Clinical Readers Sciencedirect.

FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. The September 2018 release replaces the 2010 draft guidance issued by the FDA. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry December 2019 Download the Final Guidance Document Read the Federal Register Notice Final Level 1 Guidance. .
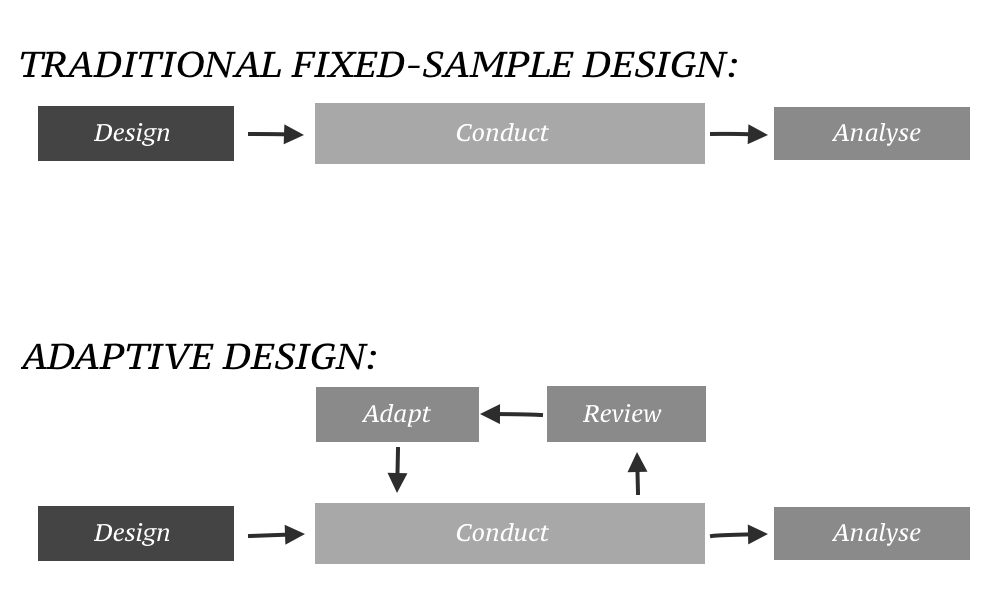
Today the Food and Drug Administration FDA issued final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design. The purpose is to. The concepts contained in this guidance are also useful for. For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. Adaptive Design Medicine Wikipedia.

The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. Office of Communication Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10903 New Hampshire Ave Bldg. Phase Ii Trials In Drug Development And Adaptive Trial Design Sciencedirect.

The September 2018 release replaces the 2010 draft guidance issued by the FDA. The September 2018 release replaces the 2010 draft guidance issued by the FDA. 51 rm2201 Silver Spring MD 20993-0002 Tel 301-796-3400. For the purposes of this guidance an adaptive design is defined as a clinical trial design that 44 allows for prospectively planned modifications to one or more aspects of the design based on. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Usa Adaptive Designs For Clinical Trials Of Drugs And Biologics Ris World.
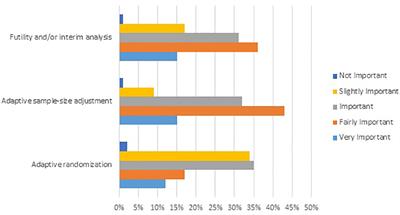
This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs. By casting dose finding as a Bayesian model selection problem we propose an adaptive design by simultaneously incorporating the toxicity and efficacy outcomes to select the optimal biological dose OBD in phase III clinical trials. Frontiers Value Of Adaptive Trials And Surrogate Endpoints For Clinical Decision Making In Rare Cancers Oncology.
In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. FDA issues guidance for industry on adaptive designs for clinical trials of drugs and biologics. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. On November 29 the Food and Drug Administration FDA issued a final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. Drug Development Simplifying The Drug Development Journey.

What is Adaptive Design Clinical Trial. For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. Office of Communication Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10903 New Hampshire Ave Bldg. What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. This guidance finalizes the draft guidance of the same title issued on October 1 2018 83 FR 49400. A Review Of Clinical Trials With An Adaptive Design And Health Economic Analysis Value In Health.

The guidance describes important principles for designing conducting and reporting the results from an adaptive clinical trial. 51 rm2201 Silver Spring MD 20993-0002 Tel 301-796-3400. Adaptive designs for exploratory clinical trials deal mainly with. For the purposes of this guidance an adaptive design is defined as a clinical trial design that 44 allows for prospectively planned modifications to one or more aspects of the design based on. What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. Short Term Effectiveness Of Biologics In Patients With Moderate To Severe Plaque Psoriasis A Systematic Review And Network Meta Analysis Journal Of Dermatological Science.

This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. Adaptive design as defined by the US. The purpose is to. FDA is a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial 1 Adaptive design characteristics include modifying an ongoing clinical trial in accordance with predetermined rules based on data from interim analyses. Have Clinical Trials In Hiv Finally Matured The Lancet Hiv.
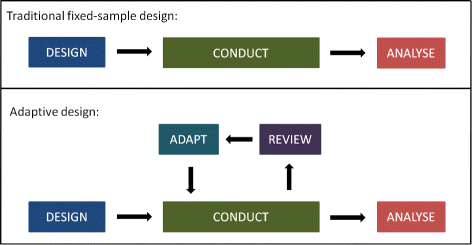
This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. FDA is a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial 1 Adaptive design characteristics include modifying an ongoing clinical trial in accordance with predetermined rules based on data from interim analyses. What is Adaptive Design Clinical Trial. FDA issues guidance for industry on adaptive designs for clinical trials of drugs and biologics. Adaptive Designs In Clinical Trials Why Use Them And How To Run And Report Them Bmc Medicine Full Text.









